Illuminating p53 function in cancer with genetically engineered mouse models
- PMID: 24394915
- PMCID: PMC4035469
- DOI: 10.1016/j.semcdb.2013.12.014
Illuminating p53 function in cancer with genetically engineered mouse models
Abstract
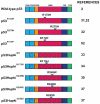
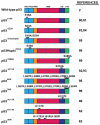
The key role of the p53 protein in tumor suppression is highlighted by its frequent mutation in human cancers and by the completely penetrant cancer predisposition of p53 null mice. Beyond providing definitive evidence for the critical function of p53 in tumor suppression, genetically engineered mouse models have offered numerous additional insights into p53 function. p53 knock-in mice expressing tumor-derived p53 mutants have revealed that these mutants display gain-of-function activities that actively promote carcinogenesis. The generation of p53 knock-in mutants with alterations in different domains of p53 has helped further elucidate the cellular and biochemical activities of p53 that are most fundamental for tumor suppression. In addition, modulation of p53 post-translational modification (PTM) status by generating p53 knock-in mouse strains with mutations in p53 PTM sites has revealed a subtlety and complexity to p53 regulation. Analyses of mouse models perturbing upstream regulators of p53 have solidified the notion that the p53 pathway can be compromised by means other than direct p53 mutation. Finally, switchable p53 models that allow p53 reactivation in tumors have helped evaluate the potential of p53 restoration therapy for cancer treatment. Collectively, mouse models have greatly enhanced our understanding of physiological p53 function and will continue to provide new biological and clinical insights in future investigations.
Keywords: Mouse models; Tumor suppression; p53.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009 May;137:413. - PubMed
-
- Purdie CA, Harrison DJ, Peter A, Dobbie L, White S, Howie SE, et al. Tumour incidence, spectrum and ploidy in mice with a large deletion in the p53 gene. Oncogene. 1994 Feb;9:603. - PubMed
-
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Current Biology: CB. 1994 Jan;4:1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

