Integrative regulation of human brain blood flow
- PMID: 24396059
- PMCID: PMC3948549
- DOI: 10.1113/jphysiol.2013.268953
Integrative regulation of human brain blood flow
Abstract
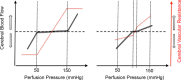
Herein, we review mechanisms regulating cerebral blood flow (CBF), with specific focus on humans. We revisit important concepts from the older literature and describe the interaction of various mechanisms of cerebrovascular control. We amalgamate this broad scope of information into a brief review, rather than detailing any one mechanism or area of research. The relationship between regulatory mechanisms is emphasized, but the following three broad categories of control are explicated: (1) the effect of blood gases and neuronal metabolism on CBF; (2) buffering of CBF with changes in blood pressure, termed cerebral autoregulation; and (3) the role of the autonomic nervous system in CBF regulation. With respect to these control mechanisms, we provide evidence against several canonized paradigms of CBF control. Specifically, we corroborate the following four key theses: (1) that cerebral autoregulation does not maintain constant perfusion through a mean arterial pressure range of 60-150 mmHg; (2) that there is important stimulatory synergism and regulatory interdependence of arterial blood gases and blood pressure on CBF regulation; (3) that cerebral autoregulation and cerebrovascular sensitivity to changes in arterial blood gases are not modulated solely at the pial arterioles; and (4) that neurogenic control of the cerebral vasculature is an important player in autoregulatory function and, crucially, acts to buffer surges in perfusion pressure. Finally, we summarize the state of our knowledge with respect to these areas, outline important gaps in the literature and suggest avenues for future research.
Figures
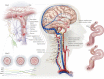
 ) and CO2 (
) and CO2 ( ), oxygen content, and proton concentration (Wolff & Lennox, ; Kontos et al. ; inlay III). The pial arteriole penetrates the pia mater through the Virchow–Robin space, where it becomes encapsulated by glial processes termed end-feet and pericytes that release vasoactive substances and respond mechanically by constricting or dilating with changes in metabolic demand of the surrounding neural matrix. Gap junctions between the endothelial and vascular smooth muscle cells allow for retrograde conductance of intramural vascular signals such that vasodilatory or constrictive signals pass to the pial arterioles. Thus, the neurovascular unit titrates blood flow to the metabolism of discrete cortical areas. Inlay III shows a qualitative schematic diagram of pial cross-sections against a hypothetical metabolic milieu spectrum. Note the vessels are not only exposed to arterial conditions but also to that of the cerebrospinal fluid that completely surrounds the pial vessel, tethered on all sides by thin processes to the pia mater. The vessels dilate with decreases in perfusion pressure, PaO2 and/or arterial O2 content (
), oxygen content, and proton concentration (Wolff & Lennox, ; Kontos et al. ; inlay III). The pial arteriole penetrates the pia mater through the Virchow–Robin space, where it becomes encapsulated by glial processes termed end-feet and pericytes that release vasoactive substances and respond mechanically by constricting or dilating with changes in metabolic demand of the surrounding neural matrix. Gap junctions between the endothelial and vascular smooth muscle cells allow for retrograde conductance of intramural vascular signals such that vasodilatory or constrictive signals pass to the pial arterioles. Thus, the neurovascular unit titrates blood flow to the metabolism of discrete cortical areas. Inlay III shows a qualitative schematic diagram of pial cross-sections against a hypothetical metabolic milieu spectrum. Note the vessels are not only exposed to arterial conditions but also to that of the cerebrospinal fluid that completely surrounds the pial vessel, tethered on all sides by thin processes to the pia mater. The vessels dilate with decreases in perfusion pressure, PaO2 and/or arterial O2 content ( ) and increases in
) and increases in  and/or [H+].
and/or [H+].

References
-
- Aaslid R, Blaha M, Sviri G, Douville CM, Newell DW. Asymmetric dynamic cerebral autoregulatory response to cyclic stimuli. Stroke. 2007;38:1465–1469. - PubMed
-
- Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. - PubMed
-
- Ainslie PN. Have a safe night: intimate protection against cerebral hyperperfusion during REM sleep. J Appl Physiol. 2009;106:1031–1033. - PubMed
-
- Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–R1495. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

