17β-estradiol inhibits mesenchymal stem cells-induced human AGS gastric cancer cell mobility via suppression of CCL5- Src/Cas/Paxillin signaling pathway
- PMID: 24396281
- PMCID: PMC3880986
- DOI: 10.7150/ijms.6851
17β-estradiol inhibits mesenchymal stem cells-induced human AGS gastric cancer cell mobility via suppression of CCL5- Src/Cas/Paxillin signaling pathway
Abstract
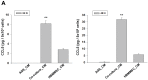
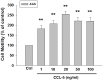
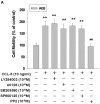
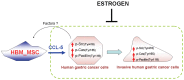
Gender differences in terms of mortality among many solid organ malignancies have been proved by epidemiological data. Estrogen has been suspected to cast a protective effect against cancer because of the lower mortality of gastric cancer in females and the benefits of hormone replacement therapy (HRT) in gastric cancer. Hence, it suggests that 17β-estradiol (E2) may affect the behavior of cancer cells. One of the key features of cancer-related mortality is metastasis. Accumulating evidences suggest that human bone marrow mesenchymal stem cells (HBMMSCs) and its secreted CCL-5 have a role in enhancing the metastatic potential of breast cancer cells. However, it is not clear whether E2 would affect HBMMSCs-induced mobility in gastric cancer cells. In this report, we show that CCL-5 secreted by HBMMSCs enhanced mobility in human AGS gastric cancer cells via activation of Src/Cas/Paxillin signaling pathway. Treatment with specific neutralizing antibody of CCL-5 significantly inhibited HBMMSCs-enhanced mobility in human AGS gastric cancer cells. We further observe that 17β-estradiol suppressed HBMMSCs-enhanced mobility by down-regulating CCL5-Src/Cas/paxillin signaling pathway in AGS cells. Collectively, these results suggest that 17β-estradiol treatment significantly inhibits HBMMSCS-induced mobility in human AGS gastric cancer cells.
Keywords: CCL-5; Cell mobility; Estrogen; Human gastric cancer cell; Mesenchymal stem cell.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005;55:74–108. - PubMed
-
- Chandanos E, Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;44:2397–403. - PubMed
-
- Frise S, Kreiger N, Gallinger S, Tomlinson G, Cotterchio M. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: findings from the canadian national enhanced cancer surveillance system. Annals of epidemiology. 2006;16:908–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

