A microfluidic device for uniform-sized cell spheroids formation, culture, harvesting and flow cytometry analysis
- PMID: 24396525
- PMCID: PMC3808411
- DOI: 10.1063/1.4824480
A microfluidic device for uniform-sized cell spheroids formation, culture, harvesting and flow cytometry analysis
Abstract
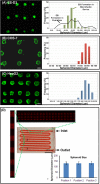
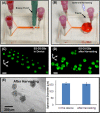
Culture of cells as three-dimensional (3D) aggregates, named spheroids, possesses great potential to improve in vitro cell models for basic biomedical research. However, such cell spheroid models are often complicated, cumbersome, and expensive compared to conventional Petri-dish cell cultures. In this work, we developed a simple microfluidic device for cell spheroid formation, culture, and harvesting. Using this device, cells could form uniformly sized spheroids due to strong cell-cell interactions and the spatial confinement of microfluidic culture chambers. We demonstrated cell spheroid formation and culture in the designed devices using embryonic stem cells, carcinoma cells, and fibroblasts. We further scaled up the device capable of simultaneously forming and culturing 5000 spheroids in a single chip. Finally, we demonstrated harvesting of the cultured spheroids from the device with a simple setup. The harvested spheroids possess great integrity, and the cells can be exploited for further flow cytometry assays due to the ample cell numbers.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
