A pneumatic valve controlled microdevice for bioanalysis
- PMID: 24396527
- PMCID: PMC3820623
- DOI: 10.1063/1.4826158
A pneumatic valve controlled microdevice for bioanalysis
Abstract
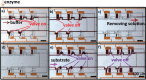
This paper describes a pneumatic valve controlled microdevice for performing mixing and reaction. This microdevice combined the degassed polydimethylsiloxane (PDMS) pumping method with a syringe-actuated valve system to control the dispensing and mixing of nanoliter solutions. The syringe was used to manually generate vacuum and to open the valves. Upon the opening of the valve, the microchamber was filled with the solution, which was driven by the external atmosphere through the degassed PDMS microchannel. With this microdevice, the enzymatic kinetics of alkaline phosphatase converting the fluorescein diphosphate was studied, and the Michaelis-Menten kinetics was analyzed. The microdevice has the advantages of simplicity and low cost in fabrication and operation.
Figures





References
-
- Ruzicka J. and Hansen E. H., Flow Injection Analysis (Wiley-Interscience, 1988).
-
- Ruzicka J. and Hansen E. H., Trend Anal. Chem. 27, 390 (2008). 10.1016/j.trac.2008.03.004 - DOI
-
- Knight J. B., Vishwanath A., Brody J. P., and Austin R. H., Phys. Rev. Lett. 80, 3863 (1998). 10.1103/PhysRevLett.80.3863 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
