An injectable, calcium responsive composite hydrogel for the treatment of acute spinal cord injury
- PMID: 24397537
- PMCID: PMC3982972
- DOI: 10.1021/am4027423
An injectable, calcium responsive composite hydrogel for the treatment of acute spinal cord injury
Abstract
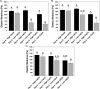
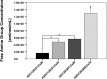
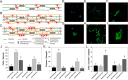
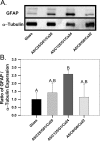
Immediately following spinal cord injury, further injury can occur through several secondary injury cascades. As a consequence of cell lysis, an increase in extracellular Ca(2+) results in additional neuronal loss by inducing apoptosis. Thus, hydrogels that reduce extracellular Ca(2+) concentration may reduce secondary injury severity. The goal of this study was to develop composite hydrogels consisting of alginate, chitosan, and genipin that interact with extracellular Ca(2+) to enable in situ gelation while maintaining an elastic modulus similar to native spinal cord (∼1000 Pa). It was hypothesized that incorporation of genipin and chitosan would regulate hydrogel electrostatic characteristics and influence hydrogel porosity, degradation, and astrocyte behavior. Hydrogel composition was varied to create hydrogels with statistically similar mechanical properties (∼1000 Pa) that demonstrated tunable charge characteristics (6-fold range in free amine concentration) and degradation rate (complete degradation between 7 and 28 days; some blends persist after 28 days). Hydrogels demonstrate high sensitivity to Ca(2+) concentration, as a 1 mM change during fabrication induced a significant change in elastic modulus. Additionally, hydrogels incubated in a Ca(2+)-containing solution exhibited an increased linear viscoelastic limit (LVE) and an increased elastic modulus above the LVE limit in a time dependent manner. An extension of the LVE limit implies a change in hydrogel cross-linking structure. Attachment assays demonstrated that addition of chitosan/genipin to alginate hydrogels induced up to a 4-fold increase in the number of attached astrocytes and facilitated astrocyte clustering on the hydrogel surface in a composition dependent manner. Furthermore, Western blots demonstrated tunable glial fibrillary acid protein (GFAP) expression in astrocytes cultured on hydrogel blends, with some hydrogel compositions demonstrating no significant increase in GFAP expression compared to astrocytes cultured on glass. Thus, alginate/chitosan/genipin hydrogel composites show promise as scaffolds that regulate astrocyte behavior and for the prevention of Ca(2+)-related secondary neuron damage during acute SCI.
Figures



References
-
- Norenberg M. D.; Smith J.; Marcillo A. J. Neurotrauma 2004, 21, 429–440. - PubMed
-
- Gilbert R. J.; Rivet C. J.; Zuidema J. M.; Popovich P. G. Crit. Rev. Biomed. Eng. 2011, 39, 125–180. - PubMed
-
- Macaya D.; Spector M. Biomed. Mater. 2012, 7, 012001. - PubMed
-
- Young W.; Yen V.; Blight A. Brain Res. 1982, 253, 105–113. - PubMed
-
- Happel R. D.; Smith K. P.; Naren Banik L.; James Powers M.; Hogan E. L.; Douglas Balentine J. Brain Res. 1981, 211, 476–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

