GSK3β, but not GSK3α, inhibits the neuronal differentiation of neural progenitor cells as a downstream target of mammalian target of rapamycin complex1
- PMID: 24397546
- PMCID: PMC4015476
- DOI: 10.1089/scd.2013.0397
GSK3β, but not GSK3α, inhibits the neuronal differentiation of neural progenitor cells as a downstream target of mammalian target of rapamycin complex1
Abstract
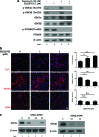
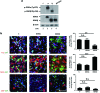
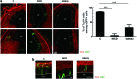
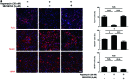
Glycogen synthase kinase 3 (GSK3) acts as an important regulator during the proliferation and differentiation of neural progenitor cells (NPCs), but the roles of the isoforms of this molecule (GSK3α and GSK3β) have not been clearly defined. In this study, we investigated the functions of GSK3α and GSK3β in the context of neuronal differentiation of murine NPCs. Treatment of primary NPCs with a GSK3 inhibitor (SB216763) resulted in an increase in the percentage of TuJ1-positive immature neurons, suggesting an inhibitory role of GSK3 in embryonic neurogenesis. Downregulation of GSK3β expression increased the percentage of TuJ1-positive cells, while knock-down of GSK3α seemed to have no effect. When primary NPCs were engineered to stably express either isoform of GSK3 using retroviral vectors, GSK3β, but not GSK3α, inhibited neuronal differentiation and helped the cells to maintain the characteristics of NPCs. Mutant GSK3β (Y216F) failed to suppress neuronal differentiation, indicating that the kinase activity of GSK3β is important for this regulatory function. Similar results were obtained in vivo when a retroviral vector expressing GSK3β was delivered to E9.5 mouse brains using the ultrasound image-guided gene delivery technique. In addition, SB216763 was found to block the rapamycin-mediated inhibition of neuronal differentiation of NPCs. Taken together, our results demonstrate that GSK3β, but not GSK3α, negatively controls the neuronal differentiation of progenitor cells and that GSK3β may act downstream of the mammalian target of rapamycin complex1 signaling pathway.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

