Controlling first-row catalysts: amination of aryl and heteroaryl chlorides and bromides with primary aliphatic amines catalyzed by a BINAP-ligated single-component Ni(0) complex
- PMID: 24397570
- PMCID: PMC3985683
- DOI: 10.1021/ja411911s
Controlling first-row catalysts: amination of aryl and heteroaryl chlorides and bromides with primary aliphatic amines catalyzed by a BINAP-ligated single-component Ni(0) complex
Erratum in
-
Correction to "Controlling First-Row Catalysts: Amination of Aryl and Heteroaryl Chlorides and Bromides with Primary Aliphatic Amines Catalyzed by a BINAP-Ligated Single-Component Ni(0) Complex".J Am Chem Soc. 2017 Mar 1;139(8):3300. doi: 10.1021/jacs.7b01463. Epub 2017 Feb 17. J Am Chem Soc. 2017. PMID: 28212008 Free PMC article. No abstract available.
Abstract
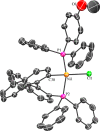
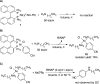
First-row metal complexes often undergo undesirable one-electron redox processes during two-electron steps of catalytic cycles. We report the amination of aryl chlorides and bromides with primary aliphatic amines catalyzed by a well-defined, single-component nickel precursor (BINAP)Ni(η(2)-NC-Ph) (BINAP = 2,2'-bis(biphenylphosphino)-1,1'-binaphthalene) that minimizes the formation of Ni(I) species and (BINAP)2Ni. The scope of the reaction encompasses electronically varied aryl chlorides and nitrogen-containing heteroaryl chlorides, including pyridine, quinoline, and isoquinoline derivatives. Mechanistic studies support the catalytic cycle involving a Ni(0)/Ni(II) couple for this nickel-catalyzed amination and are inconsistent with a Ni(I) halide intermediate. Monitoring the reaction mixture by (31)P NMR spectroscopy identified (BINAP)Ni(η(2)-NC-Ph) as the resting state of the catalyst in the amination of both aryl chlorides and bromides. Kinetic studies showed that the amination of aryl chlorides and bromides is first order in both catalyst and aryl halide and zero order in base and amine. The reaction of a representative aryl chloride is inverse first order in PhCN, but the reaction of a representative aryl bromide is zero order in PhCN. This difference in the order of the reaction in PhCN indicates that the aryl chloride reacts with (BINAP)Ni(0), formed by dissociation PhCN from (BINAP)Ni(η(2)-NC-Ph), but the aryl bromide directly reacts with (BINAP)Ni(η(2)-NC-Ph). The overall kinetic behavior is consistent with turnover-limiting oxidative addition of the aryl halide to Ni(0). Several pathways for catalyst decomposition were identified, such as the formation of the catalytically inactive bis(amine)-ligated arylnickel(II) chloride, (BINAP)2Ni(0), and the Ni(I) species [(BINAP)Ni(μ-Cl)]2. By using a well-defined nickel complex as catalyst, the formation of (BINAP)2Ni(0) is avoided and the formation of the Ni(I) species [(BINAP)Ni(μ-Cl)]2 is minimized.
Figures








References
-
- Ricci A.Modern Amination Methods; Wiley-VCH Verlag GmbH: Weinheim, 2000.
-
- Ma D.; Cai Q. Acc. Chem. Res. 2008, 41, 1450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

