Nanostructuring of biosensing electrodes with nanodiamonds for antibody immobilization
- PMID: 24397797
- PMCID: PMC4004312
- DOI: 10.1021/nn405240g
Nanostructuring of biosensing electrodes with nanodiamonds for antibody immobilization
Abstract
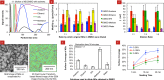
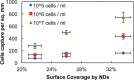
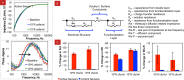
While chemical vapor deposition of diamond films is currently cost prohibitive for biosensor construction, in this paper, we show that sonication-assisted nanostructuring of biosensing electrodes with nanodiamonds (NDs) allows harnessing the hydrolytic stability of the diamond biofunctionalization chemistry for real-time continuous sensing, while improving the detector sensitivity and stability. We find that the higher surface coverages were important for improved bacterial capture and can be achieved through proper choice of solvent, ND concentration, and seeding time. A mixture of methanol and dimethyl sulfoxide provides the highest surface coverage (33.6 ± 3.4%) for the NDs with positive zeta-potential, compared to dilutions of dimethyl sulfoxide with acetone, ethanol, isopropyl alcohol, or water. Through impedance spectroscopy of ND-seeded interdigitated electrodes (IDEs), we found that the ND seeds serve as electrically conductive islands only a few nanometers apart. Also we show that the seeded NDs are amply hydrogenated to be decorated with antibodies using the UV-alkene chemistry, and higher bacterial captures can be obtained compared to our previously reported work with diamond films. When sensing bacteria from 10(6) cfu/mL E. coli O157:H7, the resistance to charge transfer at the IDEs decreased by ∼ 38.8%, which is nearly 1.5 times better than that reported previously using redox probes. Further in the case of 10(8) cfu/mL E. coli O157:H7, the charge transfer resistance changed by ∼ 46%, which is similar to the magnitude of improvement reported using magnetic nanoparticle-based sample enrichment prior to impedance detection. Thus ND seeding allows impedance biosensing in low conductivity solutions with competitive sensitivity.
Figures


Similar articles
-
A Label-Free Impedance Immunosensor Using Screen-Printed Interdigitated Electrodes and Magnetic Nanobeads for the Detection of E. coli O157:H7.Biosensors (Basel). 2015 Dec 15;5(4):791-803. doi: 10.3390/bios5040791. Biosensors (Basel). 2015. PMID: 26694478 Free PMC article.
-
Toward a Boron-Doped Ultrananocrystalline Diamond Electrode-Based Dielectrophoretic Preconcentrator.Anal Chem. 2016 Mar 1;88(5):2605-13. doi: 10.1021/acs.analchem.5b03227. Epub 2016 Feb 11. Anal Chem. 2016. PMID: 26829879
-
Interdigitated array microelectrode based impedance biosensor coupled with magnetic nanoparticle-antibody conjugates for detection of Escherichia coli O157:H7 in food samples.Biosens Bioelectron. 2007 May 15;22(11):2408-14. doi: 10.1016/j.bios.2006.08.030. Epub 2006 Oct 12. Biosens Bioelectron. 2007. PMID: 17045791
-
Nanodiamonds for bioapplications-specific targeting strategies.Biochim Biophys Acta Gen Subj. 2020 Feb;1864(2):129354. doi: 10.1016/j.bbagen.2019.04.019. Epub 2019 May 7. Biochim Biophys Acta Gen Subj. 2020. PMID: 31071412 Review.
-
Science and engineering of nanodiamond particle surfaces for biological applications (Review).Biointerphases. 2015 Sep 5;10(3):030802. doi: 10.1116/1.4927679. Biointerphases. 2015. PMID: 26245200 Review.
Cited by
-
Nanoscale Diamond-Based Formulation as an Immunomodulator and Potential Therapeutic for Lymphoma.Front Pharmacol. 2022 Apr 4;13:852065. doi: 10.3389/fphar.2022.852065. eCollection 2022. Front Pharmacol. 2022. PMID: 35444547 Free PMC article.
-
Few-Flakes Reduced Graphene Oxide Sensors for Organic Vapors with a High Signal-to-Noise Ratio.Nanomaterials (Basel). 2017 Oct 21;7(10):339. doi: 10.3390/nano7100339. Nanomaterials (Basel). 2017. PMID: 29065488 Free PMC article.
-
Recent Progresses in Nanobiosensing for Food Safety Analysis.Sensors (Basel). 2016 Jul 19;16(7):1118. doi: 10.3390/s16071118. Sensors (Basel). 2016. PMID: 27447636 Free PMC article. Review.
-
Construction of viral protein-based hybrid nanomaterials mediated by a macromolecular glue.J Mater Chem B. 2023 Aug 24;11(33):7933-7941. doi: 10.1039/d2tb02688k. J Mater Chem B. 2023. PMID: 37306104 Free PMC article.
-
Neutral Sodium Fluoride Gel Uptake of Newly Placed Nanodiamond-modified Glass Ionomers.Oral Health Prev Dent. 2020 Dec 14;18(4):1047-1053. doi: 10.3290/j.ohpd.b871065. Oral Health Prev Dent. 2020. PMID: 33499557 Free PMC article.
References
-
- Andreescu D.; Andreescu S.; Sadik O. A.. New Materials for Biosensors, Biochips and Molecular Bioelectronics. In Biosensors and Modern Biospecific Analytical Techniques; Gorton L., Ed.; Elsevier: Amsterdam, 2005; Vol. 285.
-
- Basuray S.; Senapati S.; Aijian A.; Mahon A. R.; Chang H.-C. Shear and AC Field Enhanced Carbon Nanotube Impedance Assay for Rapid, Sensitive, and Mismatch-Discriminating DNA Hybridization. ACS Nano 2009, 3, 1823–1830. - PubMed
-
- Song H. S.; Kwon O. S.; Lee S. H.; Park S. J.; Kim U.-K.; Jang J.; Park T. H. Human Taste Receptor-Functionalized Field Effect Transistor as a Human-like Nanobioelectronic Tongue. Nano Lett. 2013, 13, 172–178. - PubMed
-
- Wang M.; Wang L.; Wang G.; Ji X.; Bai Y.; Li T.; Gong S.; Li J. Application of Impedance Spectroscopy for Monitoring Colloid Au-Enhanced Antibody Immobilization and Antibody–Antigen Reactions. Biosens. Bioelectron. 2004, 19, 575–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

