Loss of long-term depression in the insular cortex after tail amputation in adult mice
- PMID: 24398034
- PMCID: PMC3912895
- DOI: 10.1186/1744-8069-10-1
Loss of long-term depression in the insular cortex after tail amputation in adult mice
Erratum in
-
Correction Notice.Mol Pain. 2017 Jan-Dec;13:1744806917711540. doi: 10.1177/1744806917711540. Mol Pain. 2017. PMID: 28478729 Free PMC article. No abstract available.
Abstract
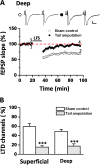
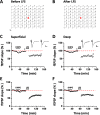
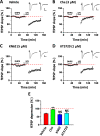
The insular cortex (IC) is an important forebrain structure involved in pain perception and taste memory formation. Using a 64-channel multi-electrode array system, we recently identified and characterized two major forms of synaptic plasticity in the adult mouse IC: long-term potentiation (LTP) and long-term depression (LTD). In this study, we investigate injury-related metaplastic changes in insular synaptic plasticity after distal tail amputation. We found that tail amputation in adult mice produced a selective loss of low frequency stimulation-induced LTD in the IC, without affecting (RS)-3,5-dihydroxyphenylglycine (DHPG)-evoked LTD. The impaired insular LTD could be pharmacologically rescued by priming the IC slices with a lower dose of DHPG application, a form of metaplasticity which involves activation of protein kinase C but not protein kinase A or calcium/calmodulin-dependent protein kinase II. These findings provide important insights into the synaptic mechanisms of cortical changes after peripheral amputation and suggest that restoration of insular LTD may represent a novel therapeutic strategy against the synaptic dysfunctions underlying the pathophysiology of phantom pain.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

