Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer
- PMID: 24398047
- PMCID: PMC3998833
- DOI: 10.1158/1078-0432.CCR-13-2332
Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer
Abstract
Purpose: We undertook this study to determine the prevalence of estrogen receptor (ER) α (ESR1) mutations throughout the natural history of hormone-dependent breast cancer and to delineate the functional roles of the most commonly detected alterations.
Experimental design: We studied a total of 249 tumor specimens from 208 patients. The specimens include 134 ER-positive (ER(+)/HER2(-)) and, as controls, 115 ER-negative (ER(-)) tumors. The ER(+) samples consist of 58 primary breast cancers and 76 metastatic samples. All tumors were sequenced to high unique coverage using next-generation sequencing targeting the coding sequence of the estrogen receptor and an additional 182 cancer-related genes.
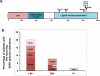
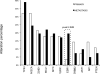
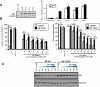
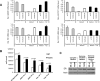
Results: Recurring somatic mutations in codons 537 and 538 within the ligand-binding domain of ER were detected in ER(+) metastatic disease. Overall, the frequency of these mutations was 12% [9/76; 95% confidence interval (CI), 6%-21%] in metastatic tumors and in a subgroup of patients who received an average of 7 lines of treatment the frequency was 20% (5/25; 95% CI, 7%-41%). These mutations were not detected in primary or treatment-naïve ER(+) cancer or in any stage of ER(-) disease. Functional studies in cell line models demonstrate that these mutations render estrogen receptor constitutive activity and confer partial resistance to currently available endocrine treatments.
Conclusions: In this study, we show evidence for the temporal selection of functional ESR1 mutations as potential drivers of endocrine resistance during the progression of ER(+) breast cancer.
©2014 AACR.
Figures
Comment in
-
Estrogen receptor mutations in breast cancer--new focus on an old target.Clin Cancer Res. 2014 Apr 1;20(7):1724-6. doi: 10.1158/1078-0432.CCR-14-0067. Epub 2014 Feb 28. Clin Cancer Res. 2014. PMID: 24583794
References
-
- Bonneterre J, Thurlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(22):3748–57. Epub 2000/11/15. PubMed PMID: 11078487. - PubMed
-
- Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(22):3758–67. Epub 2000/11/15. PubMed PMID: 11078488. - PubMed
-
- Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–9. Epub 2002/07/02. PubMed PMID: 12090977. - PubMed
-
- Garcia-Becerra R, Santos N, Diaz L, Camacho J. Mechanisms of Resistance to Endocrine Therapy in Breast Cancer: Focus on Signaling Pathways, miRNAs and Genetically Based Resistance. International journal of molecular sciences. 2012;14(1):108–45. Epub 2013/01/25. doi: 10.3390/ijms14010108. PubMed PMID: 23344024; PubMed Central PMCID: PMC3565254. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

