Evaluation of retinal nerve fiber layer thickness and axonal transport 1 and 2 weeks after 8 hours of acute intraocular pressure elevation in rats
- PMID: 24398096
- PMCID: PMC3915863
- DOI: 10.1167/iovs.13-12811
Evaluation of retinal nerve fiber layer thickness and axonal transport 1 and 2 weeks after 8 hours of acute intraocular pressure elevation in rats
Abstract
Purpose: To compare in vivo retinal nerve fiber layer thickness (RNFLT) and axonal transport at 1 and 2 weeks after an 8-hour acute IOP elevation in rats.
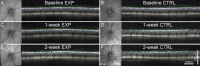
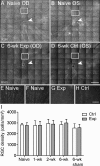
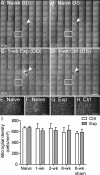
Methods: Forty-seven adult male Brown Norway rats were used. Procedures were performed under anesthesia. The IOP was manometrically elevated to 50 mm Hg or held at 15 mm Hg (sham) for 8 hours unilaterally. The RNFLT was measured by spectral-domain optical coherence tomography. Anterograde and retrograde axonal transport was assessed from confocal scanning laser ophthalmoscopy imaging 24 hours after bilateral injections of 2 μL 1% cholera toxin B-subunit conjugated to AlexaFluor 488 into the vitreous or superior colliculi, respectively. Retinal ganglion cell (RGC) and microglial densities were determined using antibodies against Brn3a and Iba-1.
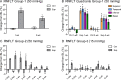
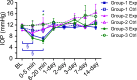
Results: The RNFLT in experimental eyes increased from baseline by 11% at 1 day (P < 0.001), peaked at 19% at 1 week (P < 0.0001), remained 11% thicker at 2 weeks (P < 0.001), recovered at 3 weeks (P > 0.05), and showed no sign of thinning at 6 weeks (P > 0.05). There was no disruption of anterograde transport at 1 week (superior colliculi fluorescence intensity, 75.3 ± 7.9 arbitrary units [AU] for the experimental eyes and 77.1 ± 6.7 AU for the control eyes) (P = 0.438) or 2 weeks (P = 0.188). There was no obstruction of retrograde transport at 1 week (RCG density, 1651 ± 153 per mm(2) for the experimental eyes and 1615 ± 135 per mm(2) for the control eyes) (P = 0.63) or 2 weeks (P = 0.25). There was no loss of Brn3a-positive RGC density at 6 weeks (P = 0.74) and no increase in microglial density (P = 0.92).
Conclusions: Acute IOP elevation to 50 mm Hg for 8 hours does not cause a persisting axonal transport deficit at 1 or 2 weeks or a detectable RNFLT or RGC loss by 6 weeks but does lead to transient RNFL thickening that resolves by 3 weeks.
Keywords: axonal transport; confocal scanning laser ophthalmoscope; glaucoma; optical coherence tomography; retinal ganglion cell; retinal nerve fiber layer.
Figures


References
-
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999; 18: 39–57 - PubMed
-
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004; 363: 1711–1720 - PubMed
-
- Emery JM, Landis D, Paton D, Boniuk M, Craig JM. The lamina cribrosa in normal and glaucomatous human eyes. Trans Am Acad Ophthalmol Otolaryngol. 1973; 78: OP290–OP297 - PubMed
-
- Vrabec F. Glaucomatous cupping of the human optic disk. Arch Klin Exp Ophthalmol. 1976; 198: 223–234 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

