Molecular evolutionary characterization of a V1R subfamily unique to strepsirrhine primates
- PMID: 24398377
- PMCID: PMC3914689
- DOI: 10.1093/gbe/evu006
Molecular evolutionary characterization of a V1R subfamily unique to strepsirrhine primates
Abstract
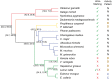
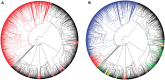
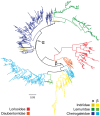
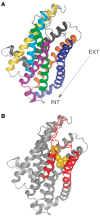
Vomeronasal receptor genes have frequently been invoked as integral to the establishment and maintenance of species boundaries among mammals due to the elaborate one-to-one correspondence between semiochemical signals and neuronal sensory inputs. Here, we report the most extensive sample of vomeronasal receptor class 1 (V1R) sequences ever generated for a diverse yet phylogenetically coherent group of mammals, the tooth-combed primates (suborder Strepsirrhini). Phylogenetic analysis confirms our intensive sampling from a single V1R subfamily, apparently unique to the strepsirrhine primates. We designate this subfamily as V1Rstrep. The subfamily retains extensive repertoires of gene copies that descend from an ancestral gene duplication that appears to have occurred prior to the diversification of all lemuriform primates excluding the basal genus Daubentonia (the aye-aye). We refer to the descendent clades as V1Rstrep-α and V1Rstrep-β. Comparison of the two clades reveals different amino acid compositions corresponding to the predicted ligand-binding site and thus potentially to altered functional profiles between the two. In agreement with previous studies of the mouse lemur (genus, Microcebus), the majority of V1Rstrep gene copies appear to be intact and under strong positive selection, particularly within transmembrane regions. Finally, despite the surprisingly high number of gene copies identified in this study, it is nonetheless probable that V1R diversity remains underestimated in these nonmodel primates and that complete characterization will be limited until high-coverage assembled genomes are available.
Keywords: G-protein-coupled receptors; chemosensory genes; gene family evolution; lemurs; olfaction; positive selection.
Figures


References
-
- Bielawski JP, Yang Z. Maximum likelihood methods for detecting adaptive evolution after gene duplication. J Struct Funct Genomics. 2003;3:201–212. - PubMed
-
- Boulet M, Crawford JC, Charpentier MJE, Drea CM. Honest olfactory ornamentation in a female-dominant primate. J Evol Biol. 2010;23:1558–1563. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

