Inhibition of OATP1B1 by tyrosine kinase inhibitors: in vitro-in vivo correlations
- PMID: 24398510
- PMCID: PMC3929889
- DOI: 10.1038/bjc.2013.811
Inhibition of OATP1B1 by tyrosine kinase inhibitors: in vitro-in vivo correlations
Erratum in
-
Inhibition of OATP1B1 by tyrosine kinase inhibitors: in vitro-in vivo correlations.Br J Cancer. 2017 Aug 22;117(5):e3. doi: 10.1038/bjc.2017.217. Epub 2017 Jul 13. Br J Cancer. 2017. PMID: 28704838 Free PMC article.
Abstract
Background: Several tyrosine kinase inhibitors (TKIs) can decrease docetaxel clearance in patients by an unknown mechanism. We hypothesised that these interactions are mediated by the hepatic uptake transporter OATP1B1.
Methods: The influence of 16 approved TKIs on transport was studied in vitro using HEK293 cells expressing OATP1B1 or its mouse equivalent Oatp1b2. Pharmacokinetic studies were performed with Oatp1b2-knockout and OATP1B1-transgenic mice.
Results: All docetaxel-interacting TKIs, including sorafenib, were identified as potent inhibitors of OATP1B1 in vitro. Although Oatp1b2 deficiency in vivo was associated with increased docetaxel exposure, single- or multiple-dose sorafenib did not influence docetaxel pharmacokinetics.
Conclusion: These findings highlight the importance of identifying proper preclinical models for verifying and predicting TKI-chemotherapy interactions involving transporters.
Figures
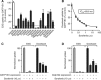
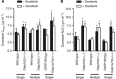
Similar articles
-
Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide.Clin Cancer Res. 2013 Mar 15;19(6):1458-66. doi: 10.1158/1078-0432.CCR-12-3306. Epub 2013 Jan 22. Clin Cancer Res. 2013. PMID: 23340295 Free PMC article.
-
Influence of polymorphic OATP1B-type carriers on the disposition of docetaxel.Clin Cancer Res. 2012 Aug 15;18(16):4433-40. doi: 10.1158/1078-0432.CCR-12-0761. Epub 2012 Jun 18. Clin Cancer Res. 2012. PMID: 22711709 Free PMC article.
-
Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance.Mol Cancer Ther. 2015 Apr;14(4):994-1003. doi: 10.1158/1535-7163.MCT-14-0547. Epub 2015 Feb 18. Mol Cancer Ther. 2015. PMID: 25695959 Free PMC article.
-
The impact of Organic Anion-Transporting Polypeptides (OATPs) on disposition and toxicity of antitumor drugs: Insights from knockout and humanized mice.Drug Resist Updat. 2016 Jul;27:72-88. doi: 10.1016/j.drup.2016.06.005. Epub 2016 Jun 25. Drug Resist Updat. 2016. PMID: 27449599 Review.
-
Clinical importance of OATP1B1 and OATP1B3 in drug-drug interactions.Drug Metab Pharmacokinet. 2011 Jun;26(3):220-7. doi: 10.2133/dmpk.DMPK-10-RV-094. Epub 2011 Feb 1. Drug Metab Pharmacokinet. 2011. PMID: 21297316 Review.
Cited by
-
Inhibition of OATP-1B1 and OATP-1B3 by tyrosine kinase inhibitors.Drug Metabol Drug Interact. 2014;29(4):249-59. doi: 10.1515/dmdi-2014-0014. Drug Metabol Drug Interact. 2014. PMID: 24807167 Free PMC article.
-
Role of Oatp2b1 in Drug Absorption and Drug-Drug Interactions.Drug Metab Dispos. 2020 May;48(5):419-425. doi: 10.1124/dmd.119.090316. Epub 2020 Feb 29. Drug Metab Dispos. 2020. PMID: 32114507 Free PMC article.
-
Influence of Tyrosine Kinase Inhibition on Organic Anion Transporting Polypeptide 1B3-Mediated Uptake.Mol Pharmacol. 2022 Jun;101(6):381-389. doi: 10.1124/molpharm.121.000287. Epub 2022 Apr 5. Mol Pharmacol. 2022. PMID: 35383108 Free PMC article.
-
Population Pharmacokinetic and Covariate Analysis of Apatinib, an Oral Tyrosine Kinase Inhibitor, in Healthy Volunteers and Patients with Solid Tumors.Clin Pharmacokinet. 2017 Jan;56(1):65-76. doi: 10.1007/s40262-016-0427-y. Clin Pharmacokinet. 2017. PMID: 27379402 Clinical Trial.
-
Hepatic uptake transporters and docetaxel disposition in mice-letter.Clin Cancer Res. 2014 Aug 1;20(15):4167. doi: 10.1158/1078-0432.CCR-14-0949. Epub 2014 Jun 11. Clin Cancer Res. 2014. PMID: 24919571 Free PMC article. No abstract available.
References
-
- Awada A, Hendlisz A, Christensen O, Lathia CD, Bartholomeus S, Lebrun F, De Valeriola D, Brendel E, Radtke M, Delaunoit T, Piccart-Gebhart M, Gil T. Phase I trial to investigate the safety, pharmacokinetics and efficacy of sorafenib combined with docetaxel in patients with advanced refractory solid tumours. Eur J Cancer. 2012;48:465–474. - PubMed
-
- Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel: recent developments. Clin Pharmacokinet. 2006;45:235–252. - PubMed
-
- Bergh J, Mariani G, Cardoso F, Liljegren A, Awada A, Viganò L, Huang X, Verkh L, Kern KA, Giorgetti C, Gianni L. Clinical and pharmacokinetic study of sunitinib and docetaxel in women with advanced breast cancer. Breast. 2012;21:507–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

