Fatty acid synthesis is inhibited by inefficient utilization of unusual fatty acids for glycerolipid assembly
- PMID: 24398521
- PMCID: PMC3903203
- DOI: 10.1073/pnas.1318511111
Fatty acid synthesis is inhibited by inefficient utilization of unusual fatty acids for glycerolipid assembly
Abstract
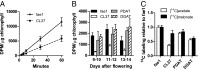
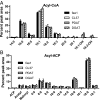
Degradation of unusual fatty acids through β-oxidation within transgenic plants has long been hypothesized as a major factor limiting the production of industrially useful unusual fatty acids in seed oils. Arabidopsis seeds expressing the castor fatty acid hydroxylase accumulate hydroxylated fatty acids up to 17% of total fatty acids in seed triacylglycerols; however, total seed oil is also reduced up to 50%. Investigations into the cause of the reduced oil phenotype through in vivo [(14)C]acetate and [(3)H]2O metabolic labeling of developing seeds surprisingly revealed that the rate of de novo fatty acid synthesis within the transgenic seeds was approximately half that of control seeds. RNAseq analysis indicated no changes in expression of fatty acid synthesis genes in hydroxylase-expressing plants. However, differential [(14)C]acetate and [(14)C]malonate metabolic labeling of hydroxylase-expressing seeds indicated the in vivo acetyl-CoA carboxylase activity was reduced to approximately half that of control seeds. Therefore, the reduction of oil content in the transgenic seeds is consistent with reduced de novo fatty acid synthesis in the plastid rather than fatty acid degradation. Intriguingly, the coexpression of triacylglycerol synthesis isozymes from castor along with the fatty acid hydroxylase alleviated the reduced acetyl-CoA carboxylase activity, restored the rate of fatty acid synthesis, and the accumulation of seed oil was substantially recovered. Together these results suggest a previously unidentified mechanism that detects inefficient utilization of unusual fatty acids within the endoplasmic reticulum and activates an endogenous pathway for posttranslational reduction of fatty acid synthesis within the plastid.
Keywords: feedback inhibition; metabolic engineering; β-oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
WRINKLED1 Rescues Feedback Inhibition of Fatty Acid Synthesis in Hydroxylase-Expressing Seeds.Plant Physiol. 2016 May;171(1):179-91. doi: 10.1104/pp.15.01906. Epub 2016 Mar 30. Plant Physiol. 2016. PMID: 27208047 Free PMC article.
-
Effects of eIFiso4G1 mutation on seed oil biosynthesis.Plant J. 2017 Jun;90(5):966-978. doi: 10.1111/tpj.13522. Epub 2017 Apr 29. Plant J. 2017. PMID: 28244172
-
Two Plastid Fatty Acid Exporters Contribute to Seed Oil Accumulation in Arabidopsis.Plant Physiol. 2020 Apr;182(4):1910-1919. doi: 10.1104/pp.19.01344. Epub 2020 Feb 4. Plant Physiol. 2020. PMID: 32019874 Free PMC article.
-
Current progress towards the metabolic engineering of plant seed oil for hydroxy fatty acids production.Plant Cell Rep. 2015 Apr;34(4):603-15. doi: 10.1007/s00299-015-1736-6. Epub 2015 Jan 11. Plant Cell Rep. 2015. PMID: 25577331 Review.
-
A multigene approach secures hydroxy fatty acid production in Arabidopsis.J Exp Bot. 2022 May 13;73(9):2875-2888. doi: 10.1093/jxb/erab533. J Exp Bot. 2022. PMID: 35560203 Review.
Cited by
-
An alternative pathway for the effective production of the omega-3 long-chain polyunsaturates EPA and ETA in transgenic oilseeds.Plant Biotechnol J. 2015 Dec;13(9):1264-75. doi: 10.1111/pbi.12328. Epub 2015 Jan 30. Plant Biotechnol J. 2015. PMID: 25640865 Free PMC article.
-
Field trial evaluation of the accumulation of omega-3 long chain polyunsaturated fatty acids in transgenic Camelina sativa: Making fish oil substitutes in plants.Metab Eng Commun. 2015 Jul 9;2:93-98. doi: 10.1016/j.meteno.2015.04.002. eCollection 2015 Dec. Metab Eng Commun. 2015. PMID: 27066395 Free PMC article.
-
PDV2 has a dosage effect on chloroplast division in Arabidopsis.Plant Cell Rep. 2017 Mar;36(3):471-480. doi: 10.1007/s00299-016-2096-6. Epub 2016 Dec 17. Plant Cell Rep. 2017. PMID: 27988788
-
14C-Tracing of Lipid Metabolism.Methods Mol Biol. 2021;2295:59-80. doi: 10.1007/978-1-0716-1362-7_5. Methods Mol Biol. 2021. PMID: 34047972
-
A Phospholipase C-Like Protein From Ricinus communis Increases Hydroxy Fatty Acids Accumulation in Transgenic Seeds of Camelina sativa.Front Plant Sci. 2018 Nov 1;9:1576. doi: 10.3389/fpls.2018.01576. eCollection 2018. Front Plant Sci. 2018. PMID: 30443260 Free PMC article.
References
-
- Badami RC, Patil KB. Structure and occurrence of unusual fatty acids in minor seed oils. Prog Lipid Res. 1980;19(3–4):119–153. - PubMed
-
- Vanhercke T, Wood CC, Stymne S, Singh SP, Green AG. Metabolic engineering of plant oils and waxes for use as industrial feedstocks. Plant Biotechnol J. 2013;11(2):197–210. - PubMed
-
- Cahoon EB, et al. Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: Solving bottlenecks in fatty acid flux. Curr Opin Plant Biol. 2007;10(3):236–244. - PubMed
-
- Napier JA. The production of unusual fatty acids in transgenic plants. Annu Rev Plant Biol. 2007;58:295–319. - PubMed
-
- Haslam RP, et al. The modification of plant oil composition via metabolic engineering—better nutrition by design. Plant Biotechnol J. 2013;11(2):157–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

