Charge segregation and low hydrophobicity are key features of ribosomal proteins from different organisms
- PMID: 24398678
- PMCID: PMC3945335
- DOI: 10.1074/jbc.M113.507707
Charge segregation and low hydrophobicity are key features of ribosomal proteins from different organisms
Abstract
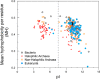
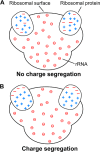
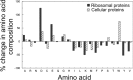
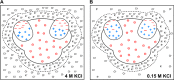
Ribosomes are large and highly charged macromolecular complexes consisting of RNA and proteins. Here, we address the electrostatic and nonpolar properties of ribosomal proteins that are important for ribosome assembly and interaction with other cellular components and may influence protein folding on the ribosome. We examined 50 S ribosomal subunits from 10 species and found a clear distinction between the net charge of ribosomal proteins from halophilic and non-halophilic organisms. We found that ∼67% ribosomal proteins from halophiles are negatively charged, whereas only up to ∼15% of ribosomal proteins from non-halophiles share this property. Conversely, hydrophobicity tends to be lower for ribosomal proteins from halophiles than for the corresponding proteins from non-halophiles. Importantly, the surface electrostatic potential of ribosomal proteins from all organisms, especially halophiles, has distinct positive and negative regions across all the examined species. Positively and negatively charged residues of ribosomal proteins tend to be clustered in buried and solvent-exposed regions, respectively. Hence, the majority of ribosomal proteins is characterized by a significant degree of intramolecular charge segregation, regardless of the organism of origin. This key property enables the ribosome to accommodate proteins within its complex scaffold regardless of their overall net charge.
Keywords: Archaea; Bacteria; Electrostatics; Hydrophobicity; Net Charge; Protein Folding; Protein Stability; Ribosomal Proteins; Ribosomes.
Figures



References
-
- Harms J., Schluenzen F., Zarivach R., Bashan A., Gat S., Agmon I., Bartels H., Franceschi F., Yonath A. (2001) High resolution structure of the large ribosomal subunit from a mesophilic eubacterium. Cell 107, 679–688 - PubMed
-
- Selmer M., Dunham C. M., Murphy F. V., 4th, Weixlbaumer A., Petry S., Kelley A. C., Weir J. R., Ramakrishnan V. (2006) Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942 - PubMed
-
- Yusupov M. M., Yusupova G. Z., Baucom A., Lieberman K., Earnest T. N., Cate J. H., Noller H. F. (2001) Crystal structure of the ribosome at 5.5 Å resolution. Science 292, 883–896 - PubMed
-
- Ramakrishnan V. (2011) Molecular biology. The eukaryotic ribosome. Science 331, 681–682 - PubMed
-
- Melnikov S., Ben-Shem A., Garreau de Loubresse N., Jenner L., Yusupova G., Yusupov M. (2012) One core, two shells: bacterial and eukaryotic ribosomes. Nat. Struct. Mol. Biol. 19, 560–567 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

