Localization and functionality of the inflammasome in neutrophils
- PMID: 24398679
- PMCID: PMC3931087
- DOI: 10.1074/jbc.M113.505636
Localization and functionality of the inflammasome in neutrophils
Abstract
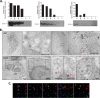
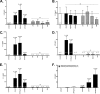
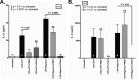
Neutrophils represent the major fraction of circulating immune cells and are rapidly recruited to sites of infection and inflammation. The inflammasome is a multiprotein complex that regulates the generation of IL-1 family proteins. The precise subcellular localization and functionality of the inflammasome in human neutrophils are poorly defined. Here we demonstrate that highly purified human neutrophils express key components of the NOD-like receptor family, pyrin domain containing 3 (NLRP3), and absent in melanoma 2 (AIM2) inflammasomes, particularly apoptosis-associated speck-like protein containing a CARD (ASC), AIM2, and caspase-1. Subcellular fractionation and microscopic analyses further showed that inflammasome components were localized in the cytoplasm and also noncanonically in secretory vesicle and tertiary granule compartments. Whereas IL-1β and IL-18 were expressed at the mRNA level and released as protein, highly purified neutrophils neither expressed nor released IL-1α at baseline or upon stimulation. Upon inflammasome activation, highly purified neutrophils released substantially lower levels of IL-1β protein compared with partially purified neutrophils. Serine proteases and caspases were differentially involved in IL-1β release, depending on the stimulus. Spontaneous activation of the NLRP3 inflammasome in neutrophils in vivo affected IL-1β, but not IL-18 release. In summary, these studies show that human neutrophils express key components of the inflammasome machinery in distinct intracellular compartments and release IL-1β and IL-18, but not IL-1α or IL-33 protein. Targeting the neutrophil inflammasome may represent a future therapeutic strategy to modulate neutrophilic inflammatory diseases, such as cystic fibrosis, rheumatoid arthritis, or sepsis.
Keywords: Cytokine; Immunity; Inflammation; Innate Immunity; Neutrophils; Pathogen-associated Molecular Pattern (PAMP); Toll-like Receptor (TLR).
Figures

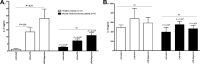
References
-
- Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 - PubMed
-
- Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 - PubMed
-
- Häger M., Cowland J. B., Borregaard N. (2010) Neutrophil granules in health and disease. J. Intern. Med. 68, 25–34 - PubMed
-
- Cassatella M. A. (1995) The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16, 21–26 - PubMed
-
- Gross O., Thomas C. J., Guarda G., Tschopp J. (2011) The inflammasome: an integrated view. Immunol. Rev. 243, 136–151 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

