Serratia marcescens suppresses host cellular immunity via the production of an adhesion-inhibitory factor against immunosurveillance cells
- PMID: 24398686
- PMCID: PMC3937657
- DOI: 10.1074/jbc.M113.544536
Serratia marcescens suppresses host cellular immunity via the production of an adhesion-inhibitory factor against immunosurveillance cells
Abstract
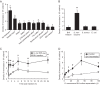
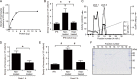
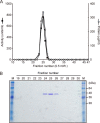
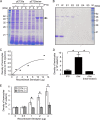
Injection of a culture supernatant of Serratia marcescens into the bloodstream of the silkworm Bombyx mori increased the number of freely circulating immunosurveillance cells (hemocytes). Using a bioassay with live silkworms, serralysin metalloprotease was purified from the culture supernatant and identified as the factor responsible for this activity. Serralysin inhibited the in vitro attachment of both silkworm hemocytes and murine peritoneal macrophages. Incubation of silkworm hemocytes or murine macrophages with serralysin resulted in degradation of the cellular immune factor BmSPH-1 or calreticulin, respectively. Furthermore, serralysin suppressed in vitro phagocytosis of bacteria by hemocytes and in vivo bacterial clearance in silkworms. Disruption of the ser gene in S. marcescens attenuated its host killing ability in silkworms and mice. These findings suggest that serralysin metalloprotease secreted by S. marcescens suppresses cellular immunity by decreasing the adhesive properties of immunosurveillance cells, thereby contributing to bacterial pathogenesis.
Keywords: Adhesion; Bacterial Toxins; Cellular Immune Response; Innate Immunity; Insect Immunity; Macrophages; Metalloprotease; Protein Purification; Silkworm.
Figures


References
-
- Hejazi A., Falkiner F. R. (1997) Serratia marcescens. J. Med. Microbiol. 46, 903–912 - PubMed
-
- Villari P., Crispino M., Salvadori A., Scarcella A. (2001) Molecular epidemiology of an outbreak of Serratia marcescens in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 22, 630–634 - PubMed
-
- Maragakis L. L., Winkler A., Tucker M. G., Cosgrove S. E., Ross T., Lawson E., Carroll K. C., Perl T. M. (2008) Outbreak of multidrug-resistant Serratia marcescens infection in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 29, 418–423 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

