A colon cancer-derived mutant of Krüppel-like factor 5 (KLF5) is resistant to degradation by glycogen synthase kinase 3β (GSK3β) and the E3 ubiquitin ligase F-box and WD repeat domain-containing 7α (FBW7α)
- PMID: 24398687
- PMCID: PMC3937667
- DOI: 10.1074/jbc.M113.508549
A colon cancer-derived mutant of Krüppel-like factor 5 (KLF5) is resistant to degradation by glycogen synthase kinase 3β (GSK3β) and the E3 ubiquitin ligase F-box and WD repeat domain-containing 7α (FBW7α)
Abstract
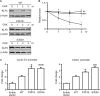
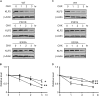
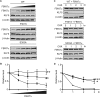
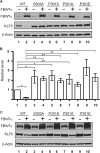
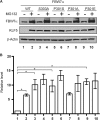
Krüppel-like factor 5 (KLF5) is a zinc finger transcription factor that is highly expressed in the crypt epithelial cells of the intestine and plays a critical role in regulating proliferation of both normal intestinal epithelial cells and colorectal cancer cells. Stability of the KLF5 is mediated by proteasomal degradation via phosphorylation by glycogen synthase kinase 3β (GSK3β) and recognition by F-box and WD repeat domain-containing 7 (FBW7) of a phosphodegron sequence surrounding serine 303 in KLF5. A genomic analysis of colorectal cancer tissues identified a somatic mutation (P301S) in KLF5 within the phosphodegron sequence. We hypothesized that due to its close proximity to the phosphodegron sequence, the P301S mutation may affect signaling that is involved in proper KLF5 degradation. We demonstrated that the P301S KLF5 mutant has a longer half-life than wild type (WT) KLF5. Furthermore, P301S KLF5 has a higher transcriptional activity than WT KLF5 as demonstrated by luciferase assays using cyclin D1 and CDC2 promoter constructs. In contrast to WT KLF5, P301S KLF5 does not physically interact with FBW7α. Concomitantly, the P301S KLF5 mutant displays reduced levels of phosphorylation at serine 303 in comparison with WT KLF5. These results of our study indicate that amino acid residue 301 of KLF5 is critical for proper recognition of the phosphodegron sequence by FBW7α and that the P301S mutation inhibits this recognition, leading to a degradation-resistant protein with elevated levels and enhanced transcriptional activity. These findings raise a potentially oncogenic role for the P301S KLF5 mutant in colorectal cancer.
Keywords: Cell Biology; Cyclin d1; Mutant; Promoters; Protein Stability; Transcription Factors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

