Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases
- PMID: 24398857
- PMCID: PMC4154371
- DOI: 10.1007/s10555-013-9484-y
Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases
Abstract
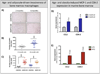
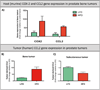
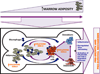
Adipocytes are important but underappreciated components of bone marrow microenvironment, and their numbers greatly increase with age, obesity, and associated metabolic pathologies. Age and obesity are also significant risk factors for development of metastatic prostate cancer. Adipocytes are metabolically active cells that secrete adipokines, growth factors, and inflammatory mediators; influence behavior and function of neighboring cells; and have a potential to disturb local milleu and dysregulate normal bone homeostasis. Increased marrow adiposity has been linked to bone marrow inflammation and osteoporosis of the bone, but its effects on growth and progression of prostate tumors that have metastasized to the skeleton are currently not known. This review focuses on fat-bone relationship in a context of normal bone homeostasis and metastatic tumor growth in bone. We discuss effects of marrow fat cells on bone metabolism, hematopoiesis, and inflammation. Special attention is given to CCL2- and COX-2-driven pathways and their potential as therapeutic targets for bone metastatic disease.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

