N-glycosylation in Haloferax volcanii: adjusting the sweetness
- PMID: 24399998
- PMCID: PMC3871713
- DOI: 10.3389/fmicb.2013.00403
N-glycosylation in Haloferax volcanii: adjusting the sweetness
Abstract
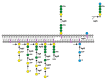
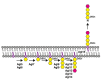
Long believed to be restricted to Eukarya, it is now known that cells of all three domains of life perform N-glycosylation, the covalent attachment of glycans to select target protein asparagine residues. Still, it is only in the last decade that pathways of N-glycosylation in Archaea have been delineated. In the haloarchaeon Haloferax volcanii, a series of Agl (archaeal glycosylation) proteins is responsible for the addition of an N-linked pentasaccharide to modified proteins, including the surface (S)-layer glycoprotein, the sole component of the surface layer surrounding the cell. The S-layer glycoprotein N-linked glycosylation profile changes, however, as a function of surrounding salinity. Upon growth at different salt concentrations, the S-layer glycoprotein is either decorated by the N-linked pentasaccharide introduced above or by both this pentasaccharide as well as a tetrasaccharide of distinct composition. Recent efforts have identified Agl5-Agl15 as components of a second Hfx. volcanii N-glycosylation pathway responsible for generating the tetrasaccharide attached to S-layer glycoprotein when growth occurs in 1.75 M but not 3.4 M NaCl-containing medium.
Keywords: Archaea; Haloferax volcanii; N-glycosylation; S-layer glycoprotein; post-translational modification; protein glycosylation.
Figures
References
-
- Abu-Qarn M., Yurist-Doutsch S., Giordano A., Trauner A., Morris H. R., Hitchen P., et al. (2007). Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J. Mol. Biol. 374 1224–1236 10.1016/j.jmb.2007.10.042 - DOI - PubMed
-
- Calo D., Guan Z., Naparstek S., Eichler J. (2011). Different routes to the same ending: Comparing the N-glycosylation processes of Haloferax volcanii and Haloarcula marismortui, two halophilic archaea from the Dead Sea. Mol. Microbiol. 81 1166–1177 10.1111/j.1365-2958.2011.07781.x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

