The extracellular phage-host interactions involved in the bacteriophage LL-H infection of Lactobacillus delbrueckii ssp. lactis ATCC 15808
- PMID: 24400001
- PMCID: PMC3870949
- DOI: 10.3389/fmicb.2013.00408
The extracellular phage-host interactions involved in the bacteriophage LL-H infection of Lactobacillus delbrueckii ssp. lactis ATCC 15808
Abstract
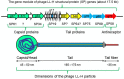
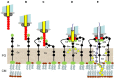
The complete genome sequence of Lactobacillus bacteriophage LL-H was determined in 1996. Accordingly, LL-H has been used as a model phage for the infection of dairy Lactobacillus, specifically for thermophilic Lactobacillus delbrueckii ssp. lactis host strains, such as ATCC 15808. One of the major goals of phage LL-H research consisted of the characterization of the first phage-host interactions at the level of phage adsorption and phage DNA injection steps to determine effective and practical methods to minimize the risks associated with the appearance and attack of phages in the manufacture of yogurt, and Swiss or Italian hard type cheeses, which typically use thermophilic lactic acid bacteria starter cultures containing L. delbrueckii strains among others. This mini review article summarizes the present data concerning (i) the special features, particle structure, and components of phage LL-H and (ii) the structure and properties of lipoteichoic acids (LTAs), which are the phage LL-H receptor components of L. delbrueckii ssp. lactis host strains. Moreover, a model of the first, extracellular, phage-host interactions for the infection of L. delbrueckii ssp. lactis ATCC 15808 by phage LL-H is presented and further discussed.
Keywords: Lactobacillus delbrueckii; antireceptor; bacteriophage LL-H; lactic acid bacteria; lipoteichoic acid; phage adsorption; phage receptor; phage–host interaction.
Figures
References
-
- Alatossava T. (1987). Molecular Biology of Lactobacillus lactis Bacteriophage LL-H (Vol. A191). Ph.D. thesis, Acta Universitatis Ouluensis, Oulu.
-
- Alatossava T., Pyhtilä M. J. (1980). Characterization of a new Lactobacillus lactis bacteriophage. IRCS Med. Sci. 8 297–298
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

