Involvement of magnitude of ambient temperature change in nonspecific effect in perceived placebo effect on lower urinary tract symptoms: study on switching of naftopidil in patients with benign prostatic hyperplasia
- PMID: 24400239
- PMCID: PMC3826939
- DOI: 10.2147/RRU.S42583
Involvement of magnitude of ambient temperature change in nonspecific effect in perceived placebo effect on lower urinary tract symptoms: study on switching of naftopidil in patients with benign prostatic hyperplasia
Abstract
Purpose: To determine if switching from one brand of the α1-adrenoceptor antagonist naftopidil (Avishot™) to another brand (Flivas™) under the same conditions causes the same changes in lower urinary tract symptoms (LUTS) and quality of life (QOL) as the perceived placebo effect, and if ambient temperature as a nonspecific factor is related to those changes in benign prostatic hyperplasia (BPH) patients.
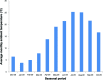
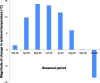
Patients and methods: A retrospective study was carried out on 217 BPH patients who had received Avishot™ for more than 6 months and then were switched to Flivas™ at the same dose and timing. The two drugs contain the same principal ingredient and display the same pharmacokinetic properties. The International Prostate Symptom Score (IPSS), QOL score, and average monthly ambient temperature at the patients' residence area from the Automated Meteorological Data Acquisition System in Japan were used for the evaluation.
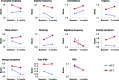
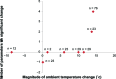
Results: A significant change in urinary storage symptoms (P = 0.006), and especially in nighttime frequency (P< 0.001), was observed by switching drugs, suggesting the perceived placebo effect. There was significant improvement of daytime frequency (P< 0.05), nighttime frequency (P< 0.001), storage symptoms (P< 0.001), and total IPSS (P< 0.05) when the magnitude of ambient temperature change from before and 3 months after switching drugs was higher than 10°C, while no significant improvement was noted in any of the parameters examined when the same was lower than 10°C.
Conclusion: The present study showed the nonspecific effect of magnitude of ambient temperature change was involved in the perceived placebo effect on LUTS, especially on storage symptoms, by switching drugs. The nonspecific effect on LUTS with BPH needs to be considered when evaluating subjective treatment efficacy of drugs for LUTS with BPH in routine clinical practice. The present study supports the lifestyle advice "avoid exposing the lower body to cold temperature" or "keep warm when it is cold" for LUTS with BPH.
Keywords: ambient temperature; benign prostatic hyperplasia; lower urinary tract symptoms; naftopidil; nonspecific effect; placebo effect.
Figures



References
-
- Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159(17):1602–1606. - PubMed
-
- Kaptchuk TJ. Powerful placebo: the dark side of the randomised controlled trial. Lancet. 1998;351:1722–1725. - PubMed
-
- Ernst E. Placebo: new insights into an old enigma. Drug Discov Today. 2007;12:413–418. - PubMed
-
- Moyad MA. The placebo effect and randomized trials: analysis of conventional medicine. Urol Clin North Am. 2002;29(1):125–133. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

