Temporal fate specification and neural progenitor competence during development
- PMID: 24400340
- PMCID: PMC3951856
- DOI: 10.1038/nrn3618
Temporal fate specification and neural progenitor competence during development
Abstract
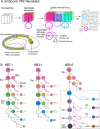
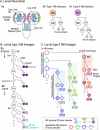
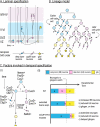
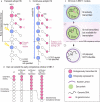
The vast diversity of neurons and glia of the CNS is generated from a small, heterogeneous population of progenitors that undergo transcriptional changes during development to sequentially specify distinct cell fates. Guided by cell-intrinsic and -extrinsic cues, invertebrate and mammalian neural progenitors carefully regulate when and how many of each cell type is produced, enabling the formation of functional neural circuits. Emerging evidence indicates that neural progenitors also undergo changes in global chromatin architecture, thereby restricting when a particular cell type can be generated. Studies of temporal-identity specification and progenitor competence can provide insight into how we could use neural progenitors to more effectively generate specific cell types for brain repair.
Figures


References
-
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature reviews. Genetics. 2000;1:20–29. doi:10.1038/35049541. - PubMed
-
- Pearson BJ, Doe CQ. Specification of temporal identity in the developing nervous system. Annu Rev Cell Dev Biol. 2004;20:619–647. doi:10.1146/annurev.cellbio.19.111301.115142. - PubMed
-
- Broadus J, et al. New neuroblast markers and the origin of the aCC/pCC neurons in the Drosophila central nervous system. Mech Dev. 1995;53:393–402. doi:0925477395004548 [pii] - PubMed
-
- Doe CQ, Technau GM. Identification and cell lineage of individual neural precursors in the Drosophila CNS. Trends Neurosci. 1993;16:510–514. - PubMed
-
- Isshiki T, Pearson B, Holbrook S, Doe CQ. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106:511–521. doi:S0092-8674(01)00465-2 [pii] - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

