Lung ultrasound in the critically ill
- PMID: 24401163
- PMCID: PMC3895677
- DOI: 10.1186/2110-5820-4-1
Lung ultrasound in the critically ill
Abstract
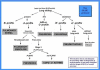
Lung ultrasound is a basic application of critical ultrasound, defined as a loop associating urgent diagnoses with immediate therapeutic decisions. It requires the mastery of ten signs: the bat sign (pleural line), lung sliding (yielding seashore sign), the A-line (horizontal artifact), the quad sign, and sinusoid sign indicating pleural effusion, the fractal, and tissue-like sign indicating lung consolidation, the B-line, and lung rockets indicating interstitial syndrome, abolished lung sliding with the stratosphere sign suggesting pneumothorax, and the lung point indicating pneumothorax. Two more signs, the lung pulse and the dynamic air bronchogram, are used to distinguish atelectasis from pneumonia. All of these disorders were assessed using CT as the "gold standard" with sensitivity and specificity ranging from 90% to 100%, allowing ultrasound to be considered as a reasonable bedside "gold standard" in the critically ill. The BLUE-protocol is a fast protocol (<3 minutes), which allows diagnosis of acute respiratory failure. It includes a venous analysis done in appropriate cases. Pulmonary edema, pulmonary embolism, pneumonia, chronic obstructive pulmonary disease, asthma, and pneumothorax yield specific profiles. Pulmonary edema, e.g., yields anterior lung rockets associated with lung sliding, making the "B-profile." The FALLS-protocol adapts the BLUE-protocol to acute circulatory failure. It makes sequential search for obstructive, cardiogenic, hypovolemic, and distributive shock using simple real-time echocardiography (right ventricle dilatation, pericardial effusion), then lung ultrasound for assessing a direct parameter of clinical volemia: the apparition of B-lines, schematically, is considered as the endpoint for fluid therapy. Other aims of lung ultrasound are decreasing medical irradiation: the LUCIFLR program (most CTs in ARDS or trauma can be postponed), a use in traumatology, intensive care unit, neonates (the signs are the same than in adults), many disciplines (pulmonology, cardiology…), austere countries, and a help in any procedure (thoracentesis). A 1992, cost-effective gray-scale unit, without Doppler, and a microconvex probe are efficient. Lung ultrasound is a holistic discipline for many reasons (e.g., one probe, perfect for the lung, is able to scan the whole-body). Its integration can provide a new definition of priorities. The BLUE-protocol and FALLS-protocol allow simplification of expert echocardiography, a clear advantage when correct cardiac windows are missing.
Figures



References
-
- Dénier A. Les ultrasons, leur application au diagnostic. Presse Med. 1946;4:307–308.
-
- Weinberger SE, Drazen JM. Harrison’s principles of internal medicine. 16. New York: McGraw-Hill; 2005. Diagnostic procedures in respiratory diseases; pp. 1505–1508.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

