Interplay between FGF21 and insulin action in the liver regulates metabolism
- PMID: 24401271
- PMCID: PMC3904602
- DOI: 10.1172/JCI67353
Interplay between FGF21 and insulin action in the liver regulates metabolism
Erratum in
-
Interplay between FGF21 and insulin action in the liver regulates metabolism.J Clin Invest. 2015 Jan;125(1):458. doi: 10.1172/JCI80223. Epub 2015 Jan 2. J Clin Invest. 2015. PMID: 25654556 Free PMC article. No abstract available.
Abstract
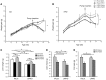
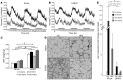
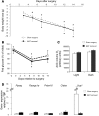
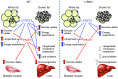
The hormone FGF21 regulates carbohydrate and lipid homeostasis as well as body weight, and increasing FGF21 improves metabolic abnormalities associated with obesity and diabetes. FGF21 is thought to act on its target tissues, including liver and adipose tissue, to improve insulin sensitivity and reduce adiposity. Here, we used mice with selective hepatic inactivation of the IR (LIRKO) to determine whether insulin sensitization in liver mediates FGF21 metabolic actions. Remarkably, hyperglycemia was completely normalized following FGF21 treatment in LIRKO mice, even though FGF21 did not reduce gluconeogenesis in these animals. Improvements in blood sugar were due in part to increased glucose uptake in brown fat, browning of white fat, and overall increased energy expenditure. These effects were preserved even after removal of the main interscapular brown fat pad. In contrast to its retained effects on reducing glucose levels, the effects of FGF21 on reducing circulating cholesterol and hepatic triglycerides and regulating the expression of key genes involved in cholesterol and lipid metabolism in liver were disrupted in LIRKO mice. Thus, FGF21 corrects hyperglycemia in diabetic mice independently of insulin action in the liver by increasing energy metabolism via activation of brown fat and browning of white fat, but intact liver insulin action is required for FGF21 to control hepatic lipid metabolism.
Figures



References
-
- Danaei G, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. - DOI - PubMed
-
- Inzucchi SE, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–1596. doi: 10.1007/s00125-012-2534-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

