Neuronal ferritin heavy chain and drug abuse affect HIV-associated cognitive dysfunction
- PMID: 24401274
- PMCID: PMC3904611
- DOI: 10.1172/JCI70090
Neuronal ferritin heavy chain and drug abuse affect HIV-associated cognitive dysfunction
Abstract
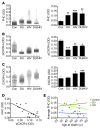
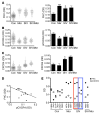
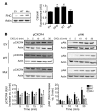
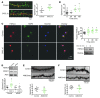
Interaction of the chemokine CXCL12 with its receptor CXCR4 promotes neuronal function and survival during embryonic development and throughout adulthood. Previous studies indicated that μ-opioid agonists specifically elevate neuronal levels of the protein ferritin heavy chain (FHC), which negatively regulates CXCR4 signaling and affects the neuroprotective function of the CXCL12/CXCR4 axis. Here, we determined that CXCL12/CXCR4 activity increased dendritic spine density, and also examined FHC expression and CXCR4 status in opiate abusers and patients with HIV-associated neurocognitive disorders (HAND), which is typically exacerbated by illicit drug use. Drug abusers and HIV patients with HAND had increased levels of FHC, which correlated with reduced CXCR4 activation, within cortical neurons. We confirmed these findings in a nonhuman primate model of SIV infection with morphine administration. Transfection of a CXCR4-expressing human cell line with an iron-deficient FHC mutant confirmed that increased FHC expression deregulated CXCR4 signaling and that this function of FHC was independent of iron binding. Furthermore, examination of morphine-treated rodents and isolated neurons expressing FHC shRNA revealed that FHC contributed to morphine-induced dendritic spine loss. Together, these data implicate FHC-dependent deregulation of CXCL12/CXCR4 as a contributing factor to cognitive dysfunction in neuroAIDS.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- T32 MH079785/MH/NIMH NIH HHS/United States
- R21 MH097623/MH/NIMH NIH HHS/United States
- U24 MH100928/MH/NIMH NIH HHS/United States
- DA32444/DA/NIDA NIH HHS/United States
- P01 DA023860/DA/NIDA NIH HHS/United States
- U01 MH083506/MH/NIMH NIH HHS/United States
- R01 DA032444/DA/NIDA NIH HHS/United States
- R01 MH085666/MH/NIMH NIH HHS/United States
- R37 DA015014/DA/NIDA NIH HHS/United States
- DA15014/DA/NIDA NIH HHS/United States
- R01 DA015014/DA/NIDA NIH HHS/United States
- U24 MH100931/MH/NIMH NIH HHS/United States
- T32-MH078795/MH/NIMH NIH HHS/United States
- MH 085666/MH/NIMH NIH HHS/United States
- P30 CA056036/CA/NCI NIH HHS/United States
- DA023860/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

