Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice
- PMID: 24401276
- PMCID: PMC3904614
- DOI: 10.1172/JCI70291
Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice
Abstract
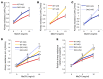
There is increasing evidence that vitamin A deficiency in utero correlates with abnormal airway smooth muscle (SM) function in postnatal life. The bioactive vitamin A metabolite retinoic acid (RA) is essential for formation of the lung primordium; however, little is known about the impact of early fetal RA deficiency on postnatal lung structure and function. Here, we provide evidence that during murine lung development, endogenous RA has a key role in restricting the airway SM differentiation program during airway formation. Using murine models of pharmacological, genetic, and dietary vitamin A/RA deficiency, we found that disruption of RA signaling during embryonic development consistently resulted in an altered airway SM phenotype with markedly increased expression of SM markers. The aberrant phenotype persisted postnatally regardless of the adult vitamin A status and manifested as structural changes in the bronchial SM and hyperresponsiveness of the airway without evidence of inflammation. Our data reveal a role for endogenous RA signaling in restricting SM differentiation and preventing precocious and excessive SM differentiation when airways are forming.
Figures

Similar articles
-
Retinoic acid reverses the airway hyperresponsiveness but not the parenchymal defect that is associated with vitamin A deficiency.Am J Physiol Lung Cell Mol Physiol. 2004 Feb;286(2):L437-44. doi: 10.1152/ajplung.00158.2003. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 14711804
-
Retinoic acid signaling is essential for airway smooth muscle homeostasis.JCI Insight. 2018 Aug 23;3(16):e120398. doi: 10.1172/jci.insight.120398. eCollection 2018 Aug 23. JCI Insight. 2018. PMID: 30135301 Free PMC article.
-
The effects of in utero vitamin D deficiency on airway smooth muscle mass and lung function.Am J Respir Cell Mol Biol. 2015 Nov;53(5):664-75. doi: 10.1165/rcmb.2014-0356OC. Am J Respir Cell Mol Biol. 2015. PMID: 25867172
-
Tidal breathing affects airway responsiveness to methacholine.Monaldi Arch Chest Dis. 2001 Dec;56(6):504-7. Monaldi Arch Chest Dis. 2001. PMID: 11980281 Review.
-
Vitamin A-retinoid signaling in pulmonary development and disease.Mol Cell Pediatr. 2016 Dec;3(1):28. doi: 10.1186/s40348-016-0054-6. Epub 2016 Aug 1. Mol Cell Pediatr. 2016. PMID: 27480876 Free PMC article. Review.
Cited by
-
Effects of AM80 compared to AC261066 in a high fat diet mouse model of liver disease.PLoS One. 2019 Jan 24;14(1):e0211071. doi: 10.1371/journal.pone.0211071. eCollection 2019. PLoS One. 2019. PMID: 30677086 Free PMC article.
-
Transmural pressure signals through retinoic acid to regulate lung branching.Development. 2022 Jan 15;149(2):dev199726. doi: 10.1242/dev.199726. Epub 2022 Jan 20. Development. 2022. PMID: 35051272 Free PMC article.
-
Diet and asthma: an update.Curr Opin Allergy Clin Immunol. 2015 Aug;15(4):369-74. doi: 10.1097/ACI.0000000000000179. Curr Opin Allergy Clin Immunol. 2015. PMID: 26110689 Free PMC article. Review.
-
Abrogation of mesenchyme-specific TGF-β signaling results in lung malformation with prenatal pulmonary cysts in mice.Am J Physiol Lung Cell Mol Physiol. 2021 Jun 1;320(6):L1158-L1168. doi: 10.1152/ajplung.00299.2020. Epub 2021 Apr 21. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33881909 Free PMC article.
-
[Effects of bacterial lysates and all trans-retinoic acid on airway inflammation in asthmatic mice].Zhongguo Dang Dai Er Ke Za Zhi. 2018 Jun;20(6):514-518. doi: 10.7499/j.issn.1008-8830.2018.06.016. Zhongguo Dang Dai Er Ke Za Zhi. 2018. PMID: 29972129 Free PMC article. Chinese.
References
-
- West KP., Jr Extent of vitamin A deficiency among preschool children and women of reproductive age. J Nutr. 2002;132(9 Suppl):2857S–2866S. - PubMed
-
- Williams SR. Nutrition and Diet Therapy: Instructor’s Manual and Text Book. Maryland Heights, Missouri, USA: Mosby; 1997.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

