A controlled study on gastrointestinal nematodes from two Swedish cattle farms showing field evidence of ivermectin resistance
- PMID: 24401545
- PMCID: PMC3892011
- DOI: 10.1186/1756-3305-7-13
A controlled study on gastrointestinal nematodes from two Swedish cattle farms showing field evidence of ivermectin resistance
Abstract
Background: Anthelmintic resistance (AR) is an increasing problem for the ruminant livestock sector worldwide. However, the extent of the problem is still relatively unknown, especially for parasitic nematodes of cattle. The effect of ivermectin (IVM) (Ivomec inj.®, Merial) was investigated in Swedish isolates of gastrointestinal nematode (GIN) populations showing signs of AR in the field to further characterise the AR status by a range of in vivo and in vitro methods.
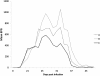
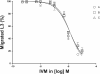
Methods: Three groups, each of 11 calves, were infected with an equal mixture of third stage larvae (L3) of Cooperia oncophora and Ostertagia ostertagi. Group A was inoculated with an IVM-susceptible laboratory isolate and groups B and C with isolates originating from 'resistant' cattle farms. Faecal egg counts (FEC) were monitored from 0 to 45 days post infection (d.p.i.), and L3 were harvested continuously for larval migration inhibition testing (LMIT) and species-specific PCR (ITS2). At 31 d.p.i., one calf from each group was necropsied and adult worms were recovered pre-treatment. At 35 d.p.i., calves from all groups were injected with IVM at the recommended dose (0.2 mg/kg bodyweight). At 45 d.p.i., another two animals from each group were sacrificed and established gastrointestinal worms were collected and counted.
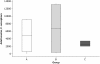
Results: A few animals in all three groups were still excreting eggs (50-150 per g faeces) 10 days post IVM injection. However, there was no significant difference in the FEC reductions in groups A (95%; 95% CI 81-99), B (98%; 92-100) and C (99%; 97-100) between 35 and 44 d.p.i. Furthermore, LMIT showed no significant difference between the three groups. Approximately 100 adult O. ostertagi were found in the abomasum of one calf (group B), whereas low to moderate numbers (400-12 200) of C. oncophora remained in the small intestine of the calves in all three groups at 45 d.p.i. PCR on L3 harvested from faecal samples up to 10 days post treatment showed a ratio of 100% C. oncophora in the calves inoculated with isolates A and B, whereas C also had 8% O. ostertagi.
Conclusions: Overall, this experiment showed that the animals were successfully treated according to the Faecal egg count reduction test (FECRT) standard (≥ 95% reduction). However, several adult worms of the dose-limiting species C. oncophora demonstrably survived the IVM treatment.
Figures
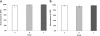
References
-
- Larsson A, Dimander SO, Waller A, Uggla A, Höglund J. A 3-year field evaluation of pasture rotation and supplementary feeding to control parasite infection in first-season grazing cattle - dynamics of pasture infectivity. Vet Parasitol. 2007;145:129–137. doi: 10.1016/j.vetpar.2006.12.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

