Self-management support using an Internet-linked tablet computer (the EDGE platform)-based intervention in chronic obstructive pulmonary disease: protocol for the EDGE-COPD randomised controlled trial
- PMID: 24401729
- PMCID: PMC3902407
- DOI: 10.1136/bmjopen-2013-004437
Self-management support using an Internet-linked tablet computer (the EDGE platform)-based intervention in chronic obstructive pulmonary disease: protocol for the EDGE-COPD randomised controlled trial
Abstract
Introduction: The potential for telehealth-based interventions to provide remote support, education and improve self-management for long-term conditions is increasingly recognised. This trial aims to determine whether an intervention delivered through an easy-to-use tablet computer can improve the quality of life of patients with chronic obstructive pulmonary disease (COPD) by providing personalised self-management information and education.
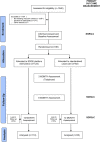
Methods and analysis: The EDGE (sElf management anD support proGrammE) for COPD is a multicentre, randomised controlled trial designed to assess the efficacy of an Internet-linked tablet computer-based intervention (the EDGE platform) in improving quality of life in patients with moderate to very severe COPD compared with usual care. Eligible patients are randomly allocated to receive the tablet computer-based intervention or usual care in a 2:1 ratio using a web-based randomisation system. Participants are recruited from respiratory outpatient clinics and pulmonary rehabilitation courses as well as from those recently discharged from hospital with a COPD-related admission and from primary care clinics. Participants allocated to the tablet computer-based intervention complete a daily symptom diary and record clinical symptoms using a Bluetooth-linked pulse oximeter. Participants allocated to receive usual care are provided with all the information given to those allocated to the intervention but without the use of the tablet computer or the facility to monitor their symptoms or physiological variables. The primary outcome of quality of life is measured using the St George's Respiratory Questionnaire for COPD patients (SGRQ-C) baseline, 6 and 12 months. Secondary outcome measures are recorded at these intervals in addition to 3 months.
Ethics and dissemination: The Research Ethics Committee for Berkshire-South Central has provided ethical approval for the conduct of the study in the recruiting regions. The results of the study will be disseminated through peer review publications and conference presentations.
Trial registration: Current controlled trials ISRCTN40367841.
Keywords: PRIMARY CARE; RESPIRATORY MEDICINE (see Thoracic Medicine).
Figures
References
-
- World Health Organization The global burden of disease: 2004 update. Geneva: WHO, 2008
-
- National Clinical Guideline Centre Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: NICE, 2010
-
- Okubadejo AA, O'Shea L, Jones PW, et al. Home assessment of activities of daily living in patients with severe chronic obstructive pulmonary disease on long-term oxygen therapy. Eur Respir J 1997;10:1572–5 - PubMed
-
- Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003;163:585–91 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical