Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer
- PMID: 24401928
- PMCID: PMC4433508
- DOI: 10.1093/annonc/mdt543
Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer
Erratum in
-
Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer.Ann Oncol. 2019 Jun 1;30(6):1017. doi: 10.1093/annonc/mdy529. Ann Oncol. 2019. PMID: 30624616 Free PMC article. No abstract available.
Abstract
Background: Nonpegylated liposomal doxorubicin liposomal doxorubicin, (Myocet™; Sopherion Therapeutics, Inc Canada, and Cephalon, Europe) (NPLD; Myocet(®)) in combination with trastuzumabHerceptin(®) (Hoffmann-La Roche) has shown promising activity and cardiac safety. We conducted a randomized phase III trial of first-line NPLD plus trastuzumab and paclitaxel (Pharmachemie B.V.) (MTP) versus trastuzumab plus paclitaxel (TP) in patients with human epidermal growth factor 2 receptor (HER2)-positive metastatic breast cancer.
Patients and methods: Patients were randomly assigned to NPLD (M, 50 mg/m(2) every 3 weeks for six cycles), trastuzumab (T, 4 mg/kg loading dose followed by 2 mg/kg weekly), and paclitaxel (P, 80 mg/m(2) weekly) or T + P at the same doses until progression or toxicity. The primary efficacy outcome was progression-free survival (PFS).
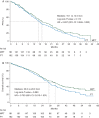
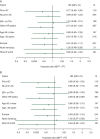
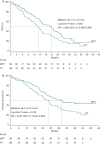
Results: One hundred and eighty-one patients were allocated to receive MTP, and 183 to TP. Median PFS was 16.1 and 14.5 months with MTP and TP, respectively [hazard ratio (HR) 0.84; two-sided P = 0.174]. In patients with estrogen receptor (ER)- and progesterone receptor (PR)-negative tumors, PFS was 20.7 and 14.0 months, respectively [HR 0.68; 95% confidence interval (CI) 0.47-0.99]. Median overall survival (OS) was 33.6 and 28.9 months with MTP and TP, respectively (HR 0.79; two-sided P = 0.083). In ER- and PR-negative tumors, OS was 38.2 and 27.9 months, respectively (HR 0.63; 95% CI 0.42-0.93). The frequency of adverse events was higher with MTP, but there was no significant difference in cardiac toxicity between treatment arms.
Conclusion(s): The trial failed to demonstrate a significant clinical improvement with the addition of M to TP regimen. The clinical benefit observed in an exploratory analysis in the ER- and PR-negative population deserves consideration for further clinical trials.
Clinical trial number: NCT00294996.
Keywords: anthracyclines, HER2, cardiac safety, Myocet, trastuzumab; breast cancer.
Figures
References
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Hudis CA Trastuzumab. Mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med. 2001;344:783–792. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

