Altered placebo and drug labeling changes the outcome of episodic migraine attacks
- PMID: 24401940
- PMCID: PMC4005597
- DOI: 10.1126/scitranslmed.3006175
Altered placebo and drug labeling changes the outcome of episodic migraine attacks
Abstract
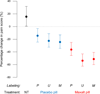
Information provided to patients is thought to influence placebo and drug effects. In a prospective, within-subjects, repeated-measures study of 66 subjects with episodic migraine, we investigated how variations in medication labeling modified placebo and drug effects. An initial attack with no treatment served as a control. In six subsequent migraine attacks, each participant received either placebo or Maxalt (10-mg rizatriptan) administered under three information conditions ranging from negative to neutral to positive (told placebo, told Maxalt or placebo, told Maxalt) (N = 459 documented attacks). Treatment order was randomized. Maxalt was superior to placebo for pain relief. When participants were given placebo labeled as (i) placebo, (ii) Maxalt or placebo, and (iii) Maxalt, the placebo effect increased progressively. Maxalt had a similar progressive boost when labeled with these three labels. The efficacies of Maxalt labeled as placebo and placebo labeled as Maxalt were similar. The efficacy of open-label placebo was superior to that of no treatment. Relative to no treatment, the placebo, under each information condition, accounted for more than 50% of the drug effect. Increasing "positive" information incrementally boosted the efficacy of both placebo and medication during migraine attacks. The benefits of placebo persisted even if placebo was honestly described. Whether treatment involves medication or placebo, the information provided to patients and the ritual of pill taking are important components of care.
Figures


References
-
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002 Oct;164:61. - PubMed
-
- Volkow ND, et al. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neurolmage. 2006 Oct 1;32:1782. - PubMed
-
- Mitchell SH, Laurent CL, de Wit H. Interaction of expectancy and the pharmacological effects of d-amphetamine: subjective effects and self-administration. Psychopharmacology (Berl) 1996 Jun;125:371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

