A blood-resistant surgical glue for minimally invasive repair of vessels and heart defects
- PMID: 24401941
- PMCID: PMC4157752
- DOI: 10.1126/scitranslmed.3006557
A blood-resistant surgical glue for minimally invasive repair of vessels and heart defects
Abstract
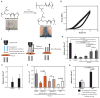
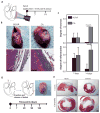
Currently, there are no clinically approved surgical glues that are nontoxic, bind strongly to tissue, and work well within wet and highly dynamic environments within the body. This is especially relevant to minimally invasive surgery that is increasingly performed to reduce postoperative complications, recovery times, and patient discomfort. We describe the engineering of a bioinspired elastic and biocompatible hydrophobic light-activated adhesive (HLAA) that achieves a strong level of adhesion to wet tissue and is not compromised by preexposure to blood. The HLAA provided an on-demand hemostatic seal, within seconds of light application, when applied to high-pressure large blood vessels and cardiac wall defects in pigs. HLAA-coated patches attached to the interventricular septum in a beating porcine heart and resisted supraphysiologic pressures by remaining attached for 24 hours, which is relevant to intracardiac interventions in humans. The HLAA could be used for many cardiovascular and surgical applications, with immediate application in repair of vascular defects and surgical hemostasis.
Figures



References
-
- Spotnitz WD. Hemostats, sealants, and adhesives: A practical guide for the surgeon. Am Surg. 2012;78:1305–1321. - PubMed
-
- Dolgin E. The sticking point. Nat Med. 2013;19:124–125. - PubMed
-
- Chang EI, Galvez MG, Glotzbach JP, Hamou CD, El-ftesi S, Rappleye CT, Sommer KM, Rajadas J, Abilez OJ, Fuller GG, Longaker MT, Gurtner GC. Vascular anastomosis using controlled phase transitions in poloxamer gels. Nat Med. 2011;17:1147–1152. - PubMed
-
- Cheema FH, Younus MJ, Roberts HG., Jr Repairing the posterior postinfarction ventricular septal defect: A left ventricular approach with a sealant reinforced multipatch technique. Semin Thorac Cardiovasc Surg. 2012;24:63–66. - PubMed
-
- Predescu D, Chaturvedi RR, Friedberg MK, Benson LN, Ozawa A, Lee KJ. Complete heart block associated with device closure of perimembranous ventricular septal defects. J Thorac Cardiovasc Surg. 2008;136:1223–1228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

