Delineating cooperative responses of processive motors in living cells
- PMID: 24402168
- PMCID: PMC3903246
- DOI: 10.1073/pnas.1313569111
Delineating cooperative responses of processive motors in living cells
Abstract
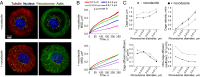
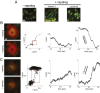
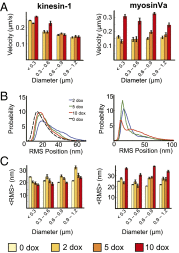
Characterizing the collective functions of cytoskeletal motors is critical to understanding mechanisms that regulate the internal organization of eukaryotic cells as well as the roles various transport defects play in human diseases. Though in vitro assays using synthetic motor complexes have generated important insights, dissecting collective motor functions within living cells still remains challenging. Here, we show that the protein heterodimerization switches FKBP-rapalog-FRB can be harnessed in engineered COS-7 cells to compare the collective responses of kinesin-1 and myosinVa motors to changes in motor number and cargo size. The dependence of cargo velocities, travel distances, and position noise on these parameters suggests that multiple myosinVa motors can cooperate more productively than collections of kinesins in COS-7 cells. In contrast to observations with kinesin-1 motors, the velocities and run lengths of peroxisomes driven by multiple myosinVa motors are found to increase with increasing motor density, but are relatively insensitive to the higher loads associated with transporting large peroxisomes in the viscoelastic environment of the COS-7 cell cytoplasm. Moreover, these distinctions appear to be derived from the different sensitivities of kinesin-1 and myosinVa velocities and detachment rates to forces at the single-motor level. The collective behaviors of certain processive motors, like myosinVa, may therefore be more readily tunable and have more substantial roles in intracellular transport regulatory mechanisms compared with those of other cytoskeletal motors.
Keywords: cooperativity; intracellular transport; microrheology; motor proteins; synthetic biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Millecamps S, Julien JP. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14(3):161–176. - PubMed
-
- Diehl MR, Zhang K, Lee HJ, Tirrell DA. Engineering cooperativity in biomotor-protein assemblies. Science. 2006;311(5766):1468–1471. - PubMed
-
- Rogers AR, Driver JW, Constantinou PE, Kenneth Jamison D, Diehl MR. Negative interference dominates collective transport of kinesin motors in the absence of load. Phys Chem Chem Phys. 2009;11(24):4882–4889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

