A STD-NMR study of the interaction of the Anabaena ferredoxin-NADP+ reductase with the coenzyme
- PMID: 24402199
- PMCID: PMC6272016
- DOI: 10.3390/molecules19010672
A STD-NMR study of the interaction of the Anabaena ferredoxin-NADP+ reductase with the coenzyme
Abstract
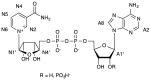
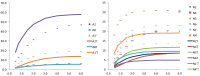
Ferredoxin-NADP+ reductase (FNR) catalyzes the electron transfer from ferredoxin to NADP+ via its flavin FAD cofactor. To get further insights in the architecture of the transient complexes produced during the hydride transfer event between the enzyme and the NADP+ coenzyme we have applied NMR spectroscopy using Saturation Transfer Difference (STD) techniques to analyze the interaction between FNRox and the oxidized state of its NADP+ coenzyme. We have found that STD NMR, together with the use of selected mutations on FNR and of the non-FNR reacting coenzyme analogue NAD+, are appropriate tools to provide further information about the the interaction epitope.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
External loops at the ferredoxin-NADP(+) reductase protein-partner binding cavity contribute to substrates allocation.Biochim Biophys Acta. 2014 Feb;1837(2):296-305. doi: 10.1016/j.bbabio.2013.11.016. Epub 2013 Dec 7. Biochim Biophys Acta. 2014. PMID: 24321506
-
A hydrogen bond network in the active site of Anabaena ferredoxin-NADP(+) reductase modulates its catalytic efficiency.Biochim Biophys Acta. 2014 Feb;1837(2):251-63. doi: 10.1016/j.bbabio.2013.10.010. Epub 2013 Nov 4. Biochim Biophys Acta. 2014. PMID: 24200908
-
The transient catalytically competent coenzyme allocation into the active site of Anabaena ferredoxin NADP+ -reductase.Eur Biophys J. 2012 Jan;41(1):117-28. doi: 10.1007/s00249-011-0704-5. Epub 2011 May 3. Eur Biophys J. 2012. PMID: 21538059
-
Structure-function relationships in Anabaena ferredoxin/ferredoxin:NADP(+) reductase electron transfer: insights from site-directed mutagenesis, transient absorption spectroscopy and X-ray crystallography.Biochim Biophys Acta. 2002 Apr 22;1554(1-2):5-21. doi: 10.1016/s0005-2728(02)00188-3. Biochim Biophys Acta. 2002. PMID: 12034466 Review.
-
Interaction and electron transfer between ferredoxin-NADP+ oxidoreductase and its partners: structural, functional, and physiological implications.Photosynth Res. 2017 Dec;134(3):265-280. doi: 10.1007/s11120-017-0372-0. Epub 2017 Mar 30. Photosynth Res. 2017. PMID: 28361449 Review.
Cited by
-
Molecular and functional characterization of ferredoxin NADP(H) oxidoreductase from Gracilaria chilensis and its complex with ferredoxin.Biol Res. 2017 Dec 8;50(1):39. doi: 10.1186/s40659-017-0144-5. Biol Res. 2017. PMID: 29221464 Free PMC article.
References
-
- Tejero J., Martínez Julvez M., Mayoral T., Luquita A., Sanz Aparicio J., Hermoso J., Hurley J., Tollin G., Gómez Moreno C., Medina M. Involvement of the pyrophosphate and the 2'-phosphate binding regions of ferredoxin-NADP+ reductase in coenzyme specificity. J. Biol. Chem. 2003;278:49203–49214. doi: 10.1074/jbc.M307934200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

