UvrD facilitates DNA repair by pulling RNA polymerase backwards
- PMID: 24402227
- PMCID: PMC4471481
- DOI: 10.1038/nature12928
UvrD facilitates DNA repair by pulling RNA polymerase backwards
Abstract
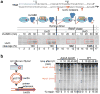
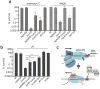
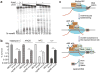
UvrD helicase is required for nucleotide excision repair, although its role in this process is not well defined. Here we show that Escherichia coli UvrD binds RNA polymerase during transcription elongation and, using its helicase/translocase activity, forces RNA polymerase to slide backward along DNA. By inducing backtracking, UvrD exposes DNA lesions shielded by blocked RNA polymerase, allowing nucleotide excision repair enzymes to gain access to sites of damage. Our results establish UvrD as a bona fide transcription elongation factor that contributes to genomic integrity by resolving conflicts between transcription and DNA repair complexes. Furthermore, we show that the elongation factor NusA cooperates with UvrD in coupling transcription to DNA repair by promoting backtracking and recruiting nucleotide excision repair enzymes to exposed lesions. Because backtracking is a shared feature of all cellular RNA polymerases, we propose that this mechanism enables RNA polymerases to function as global DNA damage scanners in bacteria and eukaryotes.
Figures





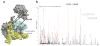





Comment in
-
Molecular biology: The tug of DNA repair.Nature. 2014 Jan 16;505(7483):298-9. doi: 10.1038/nature12850. Epub 2014 Jan 8. Nature. 2014. PMID: 24402229 No abstract available.
-
UvrD helicase: the little engine that could.Cell Cycle. 2014;13(8):1213-5. doi: 10.4161/cc.28382. Epub 2014 Mar 4. Cell Cycle. 2014. PMID: 24621500 Free PMC article. No abstract available.
References
-
- Reardon JT, Sancar A. Nucleotide excision repair. Prog Nucleic Acid Res Mol Biol. 2005;79:183–235. Medline CrossRef. - PubMed
-
- Van Houxen B, McCullough A. Nucleotide excision repair in E. coli. Ann NY Acad Sci. 1994;726:236–251. Medline CrossRef. - PubMed
-
- Ganesan A, Spivak G, Hanawalt PC. Transcription-coupled DNA repair in prokaryotes. Prog Mol Biol Transl Sci. 2012;110:25–40. Medline CrossRef. - PubMed
-
- Truglio JJ, Croteau DL, Van Houten B, Kisker C. Prokaryotic nucleotide excision repair: the UvrABC system. Chem Rev. 2006;106:233–252. Medline CrossRef. - PubMed
-
- Mellon I, Hanawalt PC. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989;342:95–98. Medline CrossRef. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

