Structural analysis of human 2'-O-ribose methyltransferases involved in mRNA cap structure formation
- PMID: 24402442
- PMCID: PMC3941023
- DOI: 10.1038/ncomms4004
Structural analysis of human 2'-O-ribose methyltransferases involved in mRNA cap structure formation
Abstract
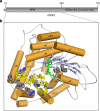
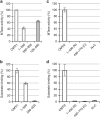
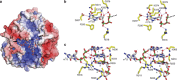
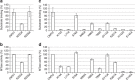
The 5' cap of human messenger RNA contains 2'-O-methylation of the first and often second transcribed nucleotide that is important for its processing, translation and stability. Human enzymes that methylate these nucleotides, termed CMTr1 and CMTr2, respectively, have recently been identified. However, the structures of these enzymes and their mechanisms of action remain unknown. In the present study, we solve the crystal structures of the active CMTr1 catalytic domain in complex with a methyl group donor and a capped oligoribonucleotide, thereby revealing the mechanism of specific recognition of capped RNA. This mechanism differs significantly from viral enzymes, thus providing a framework for their specific targeting. Based on the crystal structure of CMTr1, a comparative model of the CMTr2 catalytic domain is generated. This model, together with mutational analysis, leads to the identification of residues involved in RNA and methyl group donor binding.
Figures

References
-
- Muthukrishnan S. et al. mRNA methylation and protein synthesis in extracts from embryos of brine shrimp, Artemia salina. J. Biol. Chem. 250, 9336–9341 (1975). - PubMed
-
- Topisirovic I., Svitkin Y. V., Sonenberg N. & Shatkin A. J. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip. Rev. RNA 2, 277–298 (2011). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

