Efficient amide bond formation through a rapid and strong activation of carboxylic acids in a microflow reactor
- PMID: 24402801
- PMCID: PMC4499250
- DOI: 10.1002/anie.201307987
Efficient amide bond formation through a rapid and strong activation of carboxylic acids in a microflow reactor
Abstract
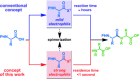
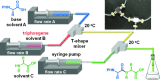
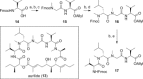
The development of highly efficient amide bond forming methods which are devoid of side reactions, including epimerization, is important, and such a method is described herein and is based on the concept of rapid and strong activation of carboxylic acids. Various carboxylic acids are rapidly (0.5 s) converted into highly active species, derived from the inexpensive and less-toxic solid triphosgene, and then rapidly (4.3 s) reacted with various amines to afford the desired peptides in high yields (74%-quant.) without significant epimerization (≤3%). Our process can be carried out at ambient temperature, and only CO2 and HCl salts of diisopropylethyl amine are generated. In the long history of peptide synthesis, a significant number of active coupling reagents have been abandoned because the highly active electrophilic species generated are usually susceptible to side reactions such as epimerization. The concept presented herein should renew interest in the use of these reagents.
Keywords: amides; amino acids; continuous flow; natural products; peptides.
Copyright © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Similar articles
-
Rapid and Mild Lactamization Using Highly Electrophilic Triphosgene in a Microflow Reactor.Chemistry. 2021 May 12;27(27):7525-7532. doi: 10.1002/chem.202100059. Epub 2021 Mar 8. Chemistry. 2021. PMID: 33496974
-
Efficient amidation from carboxylic acids and azides via selenocarboxylates: application to the coupling of amino acids and peptides with azides.J Org Chem. 2007 Feb 2;72(3):765-74. doi: 10.1021/jo061703n. J Org Chem. 2007. PMID: 17253793
-
Clickable coupling of carboxylic acids and amines at room temperature mediated by SO2F2: a significant breakthrough for the construction of amides and peptide linkages.Org Biomol Chem. 2019 Apr 17;17(16):4087-4101. doi: 10.1039/c9ob00699k. Org Biomol Chem. 2019. PMID: 30957817
-
Harnessing and engineering amide bond forming ligases for the synthesis of amides.Curr Opin Chem Biol. 2020 Apr;55:77-85. doi: 10.1016/j.cbpa.2019.12.004. Epub 2020 Feb 12. Curr Opin Chem Biol. 2020. PMID: 32058241 Review.
-
A fascinating journey into history: exploration of the world of isonitriles en route to complex amides.Angew Chem Int Ed Engl. 2012 Mar 19;51(12):2834-48. doi: 10.1002/anie.201106628. Epub 2012 Feb 24. Angew Chem Int Ed Engl. 2012. PMID: 22368033 Free PMC article. Review.
Cited by
-
Synthesis, Characterization and Evaluation of Peptide Nanostructures for Biomedical Applications.Molecules. 2021 Jul 29;26(15):4587. doi: 10.3390/molecules26154587. Molecules. 2021. PMID: 34361740 Free PMC article. Review.
-
Micro-total envelope system with silicon nanowire separator for safe carcinogenic chemistry.Nat Commun. 2016 Feb 26;7:10741. doi: 10.1038/ncomms10741. Nat Commun. 2016. PMID: 26916423 Free PMC article.
-
A decade review of triphosgene and its applications in organic reactions.Tetrahedron. 2020 Nov 20;76(47):131553. doi: 10.1016/j.tet.2020.131553. Epub 2020 Sep 6. Tetrahedron. 2020. PMID: 33883783 Free PMC article.
-
Optimization of Automated Synthesis of Amide-Linked RNA.ACS Omega. 2022 May 31;7(23):20420-20427. doi: 10.1021/acsomega.2c02742. eCollection 2022 Jun 14. ACS Omega. 2022. PMID: 35721988 Free PMC article.
-
Verification of preparations of (1H-indol-3-yl)methyl electrophiles and development of their microflow rapid generation and substitution.Commun Chem. 2023 Mar 4;6(1):47. doi: 10.1038/s42004-023-00837-1. Commun Chem. 2023. PMID: 36871078 Free PMC article.
References
-
- Yoshida J-i. Flash Chemistry—Fast Organic Synthesis in Micro Systems. Weinheim: Wiley-VCH; 2008.
-
- Yoshida J-i, Nagaki A, Yamada T. Chem. Eur. J. 2008;14:7450–7459. - PubMed
-
- Yoshida J-i. Chem. Rec. 2010;10:332–341. - PubMed
-
- For recent reviews on the formation of amide bonds, see
-
- Han SY, Kim YA. Tetrahedron. 2004;60:2447–2467.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

