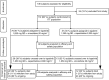
A phase II, randomized, multicenter study evaluating the combination of lapatinib and vinorelbine in women with ErbB2 overexpressing metastatic breast cancer
- PMID: 24402830
- PMCID: PMC3907671
- DOI: 10.1007/s10549-013-2828-z
A phase II, randomized, multicenter study evaluating the combination of lapatinib and vinorelbine in women with ErbB2 overexpressing metastatic breast cancer
Abstract
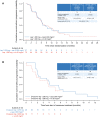
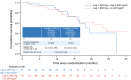
Lapatinib is approved in combination with capecitabine for treatment of patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC) who have progressed on prior trastuzumab in the metastatic setting. Vinorelbine is an important chemotherapy option for MBC. We evaluated efficacy and safety of lapatinib plus vinorelbine, compared with lapatinib plus capecitabine, in women with HER2-positive MBC. In this open-label, multicenter, phase II study, eligible patients (N = 112) were randomized 2:1 to lapatinib plus vinorelbine [(N = 75) 1,250 mg orally once daily (QD) continuously plus 20 mg/m(2)/day intravenously] or lapatinib plus capecitabine [(N = 37) 1,250 mg orally QD continuously plus 2,000 mg/m(2)/day orally, 2 doses]. The primary endpoint was progression-free survival (PFS). Other endpoints included overall survival (OS) and safety. Patients progressing within the study were given the option of crossover to the other treatment arm; time to second progression was an exploratory endpoint. Patient demographics, stratification, and prognostic factors were well balanced between treatments. Median PFS in both arms was 6.2 months [95 % confidence interval (CI) 4.2, 8.8 (lapatinib plus vinorelbine); 4.4, 8.3 (lapatinib plus capecitabine)]. Median OS on lapatinib plus vinorelbine was 24.3 months (95 % CI 16.4, NE) and 19.4 months (95 % CI 16.4, 27.2) on lapatinib plus capecitabine. In total, 42 patients opted to cross over; median PFS was 3.2 months (95 % CI 1.7, 5.1) on lapatinib plus vinorelbine and 4.0 months (95 % CI 2.1, 5.8) on lapatinib plus capecitabine. Lapatinib plus vinorelbine offers an effective treatment option for patients with HER2-overexpressing MBC, having displayed comparable efficacy and tolerability rates to lapatinib plus capecitabine.
Figures
References
-
- Hung MC, Lau YK. Basic science of HER-2/neu: a review. Semin Oncol. 1999;26:51–59. - PubMed
-
- Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. - DOI - PMC - PubMed
-
- Blackwell KL, Burstein HJ, Sledge G, Stein S, Ellis C, Casey M, Baselga J, O’Shaughnessy J. Updated survival analysis of a randomized study of lapatinib alone or in combination with tastuzumab in women with HER2-positive metastatic breast cancer progressing on trastuzumab therapy. Cancer Res. 2009;69:3. doi: 10.1158/0008-5472.SABCS-09-61. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

