Differential loss of prolyl isomerase or chaperone activity of Ran-binding protein 2 (Ranbp2) unveils distinct physiological roles of its cyclophilin domain in proteostasis
- PMID: 24403063
- PMCID: PMC3931022
- DOI: 10.1074/jbc.M113.538215
Differential loss of prolyl isomerase or chaperone activity of Ran-binding protein 2 (Ranbp2) unveils distinct physiological roles of its cyclophilin domain in proteostasis
Abstract
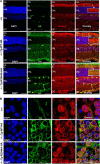
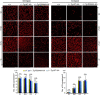
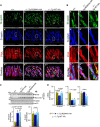
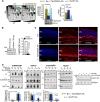
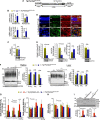
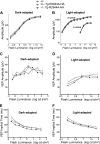
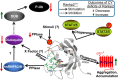
The immunophilins, cyclophilins, catalyze peptidyl cis-trans prolyl-isomerization (PPIase), a rate-limiting step in protein folding and a conformational switch in protein function. Cyclophilins are also chaperones. Noncatalytic mutations affecting the only cyclophilins with known but distinct physiological substrates, the Drosophila NinaA and its mammalian homolog, cyclophilin-B, impair opsin biogenesis and cause osteogenesis imperfecta, respectively. However, the physiological roles and substrates of most cyclophilins remain unknown. It is also unclear if PPIase and chaperone activities reflect distinct cyclophilin properties. To elucidate the physiological idiosyncrasy stemming from potential cyclophilin functions, we generated mice lacking endogenous Ran-binding protein-2 (Ranbp2) and expressing bacterial artificial chromosomes of Ranbp2 with impaired C-terminal chaperone and with (Tg-Ranbp2(WT-HA)) or without PPIase activities (Tg-Ranbp2(R2944A-HA)). The transgenic lines exhibit unique effects in proteostasis. Either line presents selective deficits in M-opsin biogenesis with its accumulation and aggregation in cone photoreceptors but without proteostatic impairment of two novel Ranbp2 cyclophilin partners, the cytokine-responsive effectors, STAT3/STAT5. Stress-induced STAT3 activation is also unaffected in Tg-Ranbp2(R2944A-HA)::Ranbp2(-/-). Conversely, proteomic analyses found that the multisystem proteinopathy/amyotrophic lateral sclerosis proteins, heterogeneous nuclear ribonucleoproteins A2/B1, are down-regulated post-transcriptionally only in Tg-Ranbp2(R2944A-HA)::Ranbp2(-/-). This is accompanied by the age- and tissue-dependent reductions of diubiquitin and ubiquitylated proteins, increased deubiquitylation activity, and accumulation of the 26 S proteasome subunits S1 and S5b. These manifestations are absent in another line, Tg-Ranbp2(CLDm-HA)::Ranbp2(-/-), harboring SUMO-1 and S1-binding mutations in the Ranbp2 cyclophilin-like domain. These results unveil distinct mechanistic and biological links between PPIase and chaperone activities of Ranbp2 cyclophilin toward proteostasis of selective substrates and with novel therapeutic potential.
Keywords: Chaperone Chaperonin; Enzymes; Proteasome; Protein Misfolding; Ubiquitination.
Figures




References
-
- Brandts J. F., Halvorson H. R., Brennan M. (1975) Consideration of the possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry 14, 4953–4963 - PubMed
-
- Fischer G., Bang H., Mech C. (1984) Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed. Biochim. Acta 43, 1101–1111 - PubMed
-
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. (1989) Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 337, 476–478 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

