Evolution of mammalian Opn5 as a specialized UV-absorbing pigment by a single amino acid mutation
- PMID: 24403072
- PMCID: PMC3924266
- DOI: 10.1074/jbc.M113.514075
Evolution of mammalian Opn5 as a specialized UV-absorbing pigment by a single amino acid mutation
Abstract
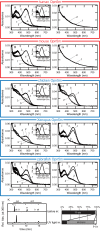
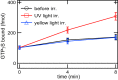
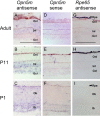
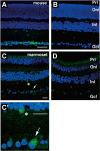
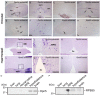
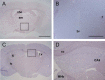
Opn5 is one of the recently identified opsin groups that is responsible for nonvisual photoreception in animals. We previously showed that a chicken homolog of mammalian Opn5 (Opn5m) is a Gi-coupled UV sensor having molecular properties typical of bistable pigments. Here we demonstrated that mammalian Opn5m evolved to be a more specialized photosensor by losing one of the characteristics of bistable pigments, direct binding of all-trans-retinal. We first confirmed that Opn5m proteins in zebrafish, Xenopus tropicalis, mouse, and human are also UV-sensitive pigments. Then we found that only mammalian Opn5m proteins lack the ability to directly bind all-trans-retinal. Mutational analysis showed that these characteristics were acquired by a single amino acid replacement at position 168. By comparing the expression patterns of Opn5m between mammals and chicken, we found that, like chicken Opn5m, mammalian Opn5m was localized in the ganglion cell layer and inner nuclear layer of the retina. However, the mouse and primate (common marmoset) opsins were distributed not in the posterior hypothalamus (including the region along the third ventricle) where chicken Opn5m is localized, but in the preoptic hypothalamus. Interestingly, RPE65, an essential enzyme for forming 11-cis-retinal in the visual cycle is expressed near the preoptic hypothalamus of the mouse and common marmoset brain but not near the region of the chicken brain where chicken Opn5m is expressed. Therefore, mammalian Opn5m may work exclusively as a short wavelength sensor in the brain as well as in the retina with the assistance of an 11-cis-retinal-supplying system.
Keywords: G Proteins; Molecular Evolution; Nonvisual Photoreception; Photoreceptors; Rhodopsin; Signal Transduction.
Figures




Similar articles
-
Two UV-Sensitive Photoreceptor Proteins, Opn5m and Opn5m2 in Ray-Finned Fish with Distinct Molecular Properties and Broad Distribution in the Retina and Brain.PLoS One. 2016 May 11;11(5):e0155339. doi: 10.1371/journal.pone.0155339. eCollection 2016. PLoS One. 2016. PMID: 27167972 Free PMC article.
-
Amino acid residue at position 188 determines the UV-sensitive bistable property of vertebrate non-visual opsin Opn5.Commun Biol. 2022 Jan 18;5(1):63. doi: 10.1038/s42003-022-03010-x. Commun Biol. 2022. PMID: 35042952 Free PMC article.
-
Unexpected molecular diversity of vertebrate nonvisual opsin Opn5.Biophys Rev. 2020 Apr;12(2):333-338. doi: 10.1007/s12551-020-00654-z. Epub 2020 Mar 9. Biophys Rev. 2020. PMID: 32152922 Free PMC article. Review.
-
Localization of the ultraviolet-sensor Opn5m and its effect on myopia-related gene expression in the late-embryonic chick eye.Biochem Biophys Rep. 2019 Jul 19;19:100665. doi: 10.1016/j.bbrep.2019.100665. eCollection 2019 Sep. Biochem Biophys Rep. 2019. PMID: 31463372 Free PMC article.
-
Molecular Property, Manipulation, and Potential Use of Opn5 and Its Homologs.J Mol Biol. 2024 Mar 1;436(5):168319. doi: 10.1016/j.jmb.2023.168319. Epub 2023 Oct 20. J Mol Biol. 2024. PMID: 37865286 Review.
Cited by
-
Characterisation of light responses in the retina of mice lacking principle components of rod, cone and melanopsin phototransduction signalling pathways.Sci Rep. 2016 Jun 15;6:28086. doi: 10.1038/srep28086. Sci Rep. 2016. PMID: 27301998 Free PMC article.
-
Circadian Oscillations in the Murine Preoptic Area Are Reset by Temperature, but Not Light.Front Physiol. 2022 Jul 22;13:934591. doi: 10.3389/fphys.2022.934591. eCollection 2022. Front Physiol. 2022. PMID: 35957988 Free PMC article.
-
Selective optogenetic control of Gq signaling using human Neuropsin.Nat Commun. 2022 Apr 1;13(1):1765. doi: 10.1038/s41467-022-29265-w. Nat Commun. 2022. PMID: 35365606 Free PMC article.
-
Whole genome assembly of the armored loricariid catfish Ancistrus triradiatus highlights herbivory signatures.Mol Genet Genomics. 2022 Nov;297(6):1627-1642. doi: 10.1007/s00438-022-01947-6. Epub 2022 Aug 25. Mol Genet Genomics. 2022. PMID: 36006456 Free PMC article.
-
Analysis of the opsin repertoire in the tardigrade Hypsibius dujardini provides insights into the evolution of opsin genes in panarthropoda.Genome Biol Evol. 2014 Sep 4;6(9):2380-91. doi: 10.1093/gbe/evu193. Genome Biol Evol. 2014. PMID: 25193307 Free PMC article.
References
-
- Koyanagi M., Terakita A. (2008) Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem. Photobiol. 84, 1024–1030 - PubMed
-
- Tarttelin E. E., Bellingham J., Hankins M. W., Foster R. G., Lucas R. J. (2003) Neuropsin (Opn5). A novel opsin identified in mammalian neural tissue. FEBS Lett. 554, 410–416 - PubMed
-
- Tomonari S., Migita K., Takagi A., Noji S., Ohuchi H. (2008) Expression patterns of the opsin 5-related genes in the developing chicken retina. Dev. Dyn. 237, 1910–1922 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

