Mutant LRRK2 toxicity in neurons depends on LRRK2 levels and synuclein but not kinase activity or inclusion bodies
- PMID: 24403142
- PMCID: PMC3870929
- DOI: 10.1523/JNEUROSCI.2712-13.2014
Mutant LRRK2 toxicity in neurons depends on LRRK2 levels and synuclein but not kinase activity or inclusion bodies
Abstract
By combining experimental neuron models and mathematical tools, we developed a "systems" approach to deconvolve cellular mechanisms of neurodegeneration underlying the most common known cause of Parkinson's disease (PD), mutations in leucine-rich repeat kinase 2 (LRRK2). Neurons ectopically expressing mutant LRRK2 formed inclusion bodies (IBs), retracted neurites, accumulated synuclein, and died prematurely, recapitulating key features of PD. Degeneration was predicted from the levels of diffuse mutant LRRK2 that each neuron contained, but IB formation was neither necessary nor sufficient for death. Genetic or pharmacological blockade of its kinase activity destabilized LRRK2 and lowered its levels enough to account for the moderate reduction in LRRK2 toxicity that ensued. By contrast, targeting synuclein, including neurons made from PD patient-derived induced pluripotent cells, dramatically reduced LRRK2-dependent neurodegeneration and LRRK2 levels. These findings suggest that LRRK2 levels are more important than kinase activity per se in predicting toxicity and implicate synuclein as a major mediator of LRRK2-induced neurodegeneration.
Keywords: LRRK2; Parkinson's disease; mechanisms; single cell; synuclein.
Figures

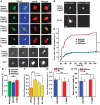
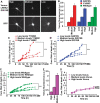

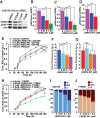



References
-
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lackingα-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/S0896-6273(00)80886-7. - DOI - PubMed
-
- Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. - DOI - PMC - PubMed
-
- Andersen PK, Gill RD. Cox's regression model for counting processes, a large sample study. Ann Statistics. 1982;10:1100–1120. doi: 10.1214/aos/1176345976. - DOI
-
- Andres-Mateos E, Mejias R, Sasaki M, Li X, Lin BM, Biskup S, Zhang L, Banerjee R, Thomas B, Yang L, Liu G, Beal MF, Huso DL, Dawson TM, Dawson VL. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) J Neurosci. 2009;29:15846–15850. doi: 10.1523/JNEUROSCI.4357-09.2009. - DOI - PMC - PubMed
-
- Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, Bolam JP, Chandra SS, Cragg SJ, Wade-Martins R, Buchman VL. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci. 2011;31:7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2P01 AG0224074/AG/NIA NIH HHS/United States
- R01 NS039074/NS/NINDS NIH HHS/United States
- U24 NS078370/NS/NINDS NIH HHS/United States
- R01 NS083390/NS/NINDS NIH HHS/United States
- RR18928/RR/NCRR NIH HHS/United States
- P30 NS069496/NS/NINDS NIH HHS/United States
- Wellcome Trust/United Kingdom
- 1K08NS062954-01A1/NS/NINDS NIH HHS/United States
- 3R01 NS039074/NS/NINDS NIH HHS/United States
- P30NS069496/NS/NINDS NIH HHS/United States
- 2R01 NS04549/NS/NINDS NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- K08 NS062954/NS/NINDS NIH HHS/United States
- C06 RR018928/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
