Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control
- PMID: 24403146
- PMCID: PMC3870931
- DOI: 10.1523/JNEUROSCI.3315-13.2014
Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control
Abstract
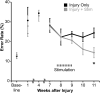
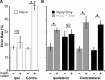
Partial injury to the corticospinal tract (CST) causes sprouting of intact axons at their targets, and this sprouting correlates with functional improvement. Electrical stimulation of motor cortex augments sprouting of intact CST axons and promotes functional recovery when applied soon after injury. We hypothesized that electrical stimulation of motor cortex in the intact hemisphere after chronic lesion of the CST in the other hemisphere would restore function through ipsilateral control. To test motor skill, rats were trained and tested to walk on a horizontal ladder with irregularly spaced rungs. Eight weeks after injury, produced by pyramidal tract transection, half of the rats received forelimb motor cortex stimulation of the intact hemisphere. Rats with injury and stimulation had significantly improved forelimb control compared with rats with injury alone and achieved a level of proficiency similar to uninjured rats. To test whether recovery of forelimb function was attributable to ipsilateral control, we selectively inactivated the stimulated motor cortex using the GABA agonist muscimol. The dose of muscimol we used produces strong contralateral but no ipsilateral impairments in naive rats. In rats with injury and stimulation, but not those with injury alone, inactivation caused worsening of forelimb function; the initial deficit was reinstated. These results demonstrate that electrical stimulation can promote recovery of motor function when applied late after injury and that motor control can be exerted from the ipsilateral motor cortex. These results suggest that the uninjured motor cortex could be targeted for brain stimulation in people with large unilateral CST lesions.
Figures

Similar articles
-
Plasticity in One Hemisphere, Control From Two: Adaptation in Descending Motor Pathways After Unilateral Corticospinal Injury in Neonatal Rats.Front Neural Circuits. 2018 Apr 12;12:28. doi: 10.3389/fncir.2018.00028. eCollection 2018. Front Neural Circuits. 2018. PMID: 29706871 Free PMC article.
-
Combined motor cortex and spinal cord neuromodulation promotes corticospinal system functional and structural plasticity and motor function after injury.Exp Neurol. 2016 Mar;277:46-57. doi: 10.1016/j.expneurol.2015.12.008. Epub 2015 Dec 18. Exp Neurol. 2016. PMID: 26708732 Free PMC article.
-
Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth.J Neurosci. 2010 Aug 11;30(32):10918-26. doi: 10.1523/JNEUROSCI.1435-10.2010. J Neurosci. 2010. PMID: 20702720 Free PMC article.
-
Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function.Front Integr Neurosci. 2014 Jun 18;8:51. doi: 10.3389/fnint.2014.00051. eCollection 2014. Front Integr Neurosci. 2014. PMID: 24994971 Free PMC article. Review.
-
Preclinical and Clinical Evidence on Ipsilateral Corticospinal Projections: Implication for Motor Recovery.Transl Stroke Res. 2017 Dec;8(6):529-540. doi: 10.1007/s12975-017-0551-5. Epub 2017 Jul 9. Transl Stroke Res. 2017. PMID: 28691140 Free PMC article. Review.
Cited by
-
Diabetes Mellitus-Related Dysfunction of the Motor System.Int J Mol Sci. 2020 Oct 11;21(20):7485. doi: 10.3390/ijms21207485. Int J Mol Sci. 2020. PMID: 33050583 Free PMC article. Review.
-
Rewiring the Lesioned Brain: Electrical Stimulation for Post-Stroke Motor Restoration.J Stroke. 2020 Jan;22(1):47-63. doi: 10.5853/jos.2019.03027. Epub 2020 Jan 31. J Stroke. 2020. PMID: 32027791 Free PMC article. Review.
-
Skilled Bimanual Training Drives Motor Cortex Plasticity in Children With Unilateral Cerebral Palsy.Neurorehabil Neural Repair. 2016 Oct;30(9):834-44. doi: 10.1177/1545968315625838. Epub 2016 Feb 11. Neurorehabil Neural Repair. 2016. PMID: 26867559 Free PMC article. Clinical Trial.
-
Transcranial direct current stimulation (tDCS) paired with massed practice training to promote adaptive plasticity and motor recovery in chronic incomplete tetraplegia: A pilot study.J Spinal Cord Med. 2018 Sep;41(5):503-517. doi: 10.1080/10790268.2017.1361562. Epub 2017 Aug 7. J Spinal Cord Med. 2018. PMID: 28784042 Free PMC article. Clinical Trial.
-
Neuromodulation technologies improve functional recovery after brain injury: From bench to bedside.Neural Regen Res. 2026 Feb 1;21(2):506-520. doi: 10.4103/NRR.NRR-D-24-00652. Epub 2024 Dec 7. Neural Regen Res. 2026. PMID: 39851132 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
