Axon regeneration genes identified by RNAi screening in C. elegans
- PMID: 24403161
- PMCID: PMC3870940
- DOI: 10.1523/JNEUROSCI.3859-13.2014
Axon regeneration genes identified by RNAi screening in C. elegans
Abstract
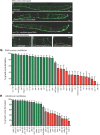
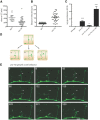
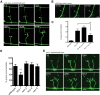
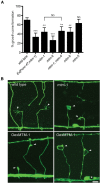
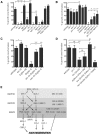
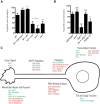
Axons of the mammalian CNS lose the ability to regenerate soon after development due to both an inhibitory CNS environment and the loss of cell-intrinsic factors necessary for regeneration. The complex molecular events required for robust regeneration of mature neurons are not fully understood, particularly in vivo. To identify genes affecting axon regeneration in Caenorhabditis elegans, we performed both an RNAi-based screen for defective motor axon regeneration in unc-70/β-spectrin mutants and a candidate gene screen. From these screens, we identified at least 50 conserved genes with growth-promoting or growth-inhibiting functions. Through our analysis of mutants, we shed new light on certain aspects of regeneration, including the role of β-spectrin and membrane dynamics, the antagonistic activity of MAP kinase signaling pathways, and the role of stress in promoting axon regeneration. Many gene candidates had not previously been associated with axon regeneration and implicate new pathways of interest for therapeutic intervention.
Keywords: C. elegans; DLK; MAP kinases; axon regeneration; laser axotomy; neural regeneration.
Figures
References
-
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012;31:1350–1363. doi: 10.1038/emboj.2011.494. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
