Comparison of sleep spindles and theta oscillations in the hippocampus
- PMID: 24403164
- PMCID: PMC3870943
- DOI: 10.1523/JNEUROSCI.0552-13.2014
Comparison of sleep spindles and theta oscillations in the hippocampus
Abstract
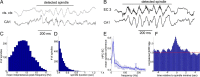
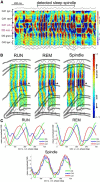
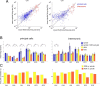
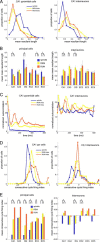
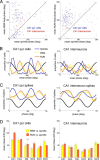
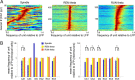
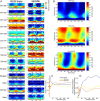
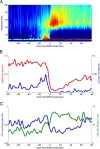
Several network patterns allow for information exchange between the neocortex and the entorhinal-hippocampal complex, including theta oscillations and sleep spindles. How neurons are organized in these respective patterns is not well understood. We examined the cellular-synaptic generation of sleep spindles and theta oscillations in the waking rat and during rapid eye movement (REM) sleep by simultaneously recording local field and spikes in the regions and layers of the hippocampus and entorhinal cortex (EC). We show the following: (1) current source density analysis reveals that similar anatomical substrates underlie spindles and theta in the hippocampus, although the hippocampal subregions are more synchronized during spindles than theta; (2) the spiking of putative principal cells and interneurons in the CA1, CA3, and dentate gyrus subregions of the hippocampus, as well as layers 2, 3, and 5 of medial EC, are significantly phase locked to spindles detected in CA1; (3) the relationship between local field potential (LFP) phase and unit spiking differs between spindles and theta; (4) individual hippocampal principal cells generally do not fire in a rhythmic manner during spindles; (5) power in gamma (30-90 Hz) and epsilon (>90 Hz) bands of hippocampal LFP is modulated by the phase of spindle oscillations; and (6) unit firing rates during spindles were not significantly affected by whether spindles occurred during non-REM or transitions between non-REM and REM sleep. Thus, despite the similar current generator inputs and macroscopic appearance of the LFP, the organization of neuronal firing patterns during spindles bears little resemblance to that of theta oscillations.
Keywords: entorhinal cortex; gamma; hippocampus; sleep; spindle; theta.
Figures
Similar articles
-
Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep.J Neurosci. 2008 Jun 25;28(26):6731-41. doi: 10.1523/JNEUROSCI.1227-08.2008. J Neurosci. 2008. PMID: 18579747 Free PMC article.
-
Theta oscillations decrease spike synchrony in the hippocampus and entorhinal cortex.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 23;369(1635):20120530. doi: 10.1098/rstb.2012.0530. Print 2014 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24366139 Free PMC article.
-
Electrophysiological Evidence That the Retrosplenial Cortex Displays a Strong and Specific Activation Phased with Hippocampal Theta during Paradoxical (REM) Sleep.J Neurosci. 2017 Aug 16;37(33):8003-8013. doi: 10.1523/JNEUROSCI.0026-17.2017. Epub 2017 Jul 20. J Neurosci. 2017. PMID: 28729438 Free PMC article.
-
Hippocampal information processing across sleep/wake cycles.Neurosci Res. 2017 May;118:30-47. doi: 10.1016/j.neures.2017.04.018. Epub 2017 May 12. Neurosci Res. 2017. PMID: 28506629 Review.
-
Brain neural patterns and the memory function of sleep.Science. 2021 Oct 29;374(6567):560-564. doi: 10.1126/science.abi8370. Epub 2021 Oct 28. Science. 2021. PMID: 34709916 Free PMC article. Review.
Cited by
-
Relating Pupil Diameter and Blinking to Cortical Activity and Hemodynamics across Arousal States.J Neurosci. 2023 Feb 8;43(6):949-964. doi: 10.1523/JNEUROSCI.1244-22.2022. Epub 2022 Dec 14. J Neurosci. 2023. PMID: 36517240 Free PMC article.
-
The Evolving Role of Animal Models in the Discovery and Development of Novel Treatments for Psychiatric Disorders.Adv Neurobiol. 2023;30:37-99. doi: 10.1007/978-3-031-21054-9_3. Adv Neurobiol. 2023. PMID: 36928846
-
Low Activity Microstates During Sleep.Sleep. 2017 Jun 1;40(6):zsx066. doi: 10.1093/sleep/zsx066. Sleep. 2017. PMID: 28431164 Free PMC article.
-
Imaging high-frequency voltage dynamics in multiple neuron classes of behaving mammals.bioRxiv [Preprint]. 2024 Aug 16:2024.08.15.607428. doi: 10.1101/2024.08.15.607428. bioRxiv. 2024. Update in: Cell. 2025 Aug 7;188(16):4401-4423.e31. doi: 10.1016/j.cell.2025.06.028. PMID: 39185175 Free PMC article. Updated. Preprint.
-
Spatial information enhanced by non-spatial information in hippocampal granule cells.Cogn Neurodyn. 2015 Feb;9(1):1-12. doi: 10.1007/s11571-014-9309-x. Epub 2014 Sep 11. Cogn Neurodyn. 2015. PMID: 26052358 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
