BAY 87-2243, a highly potent and selective inhibitor of hypoxia-induced gene activation has antitumor activities by inhibition of mitochondrial complex I
- PMID: 24403227
- PMCID: PMC3892793
- DOI: 10.1002/cam4.112
BAY 87-2243, a highly potent and selective inhibitor of hypoxia-induced gene activation has antitumor activities by inhibition of mitochondrial complex I
Abstract
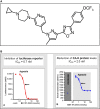
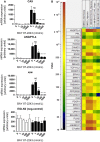
The activation of the transcription factor hypoxia-inducible factor-1 (HIF-1) plays an essential role in tumor development, tumor progression, and resistance to chemo- and radiotherapy. In order to identify compounds targeting the HIF pathway, a small molecule library was screened using a luciferase-driven HIF-1 reporter cell line under hypoxia. The high-throughput screening led to the identification of a class of aminoalkyl-substituted compounds that inhibited hypoxia-induced HIF-1 target gene expression in human lung cancer cell lines at low nanomolar concentrations. Lead structure BAY 87-2243 was found to inhibit HIF-1α and HIF-2α protein accumulation under hypoxic conditions in non-small cell lung cancer (NSCLC) cell line H460 but had no effect on HIF-1α protein levels induced by the hypoxia mimetics desferrioxamine or cobalt chloride. BAY 87-2243 had no effect on HIF target gene expression levels in RCC4 cells lacking Von Hippel-Lindau (VHL) activity nor did the compound affect the activity of HIF prolyl hydroxylase-2. Antitumor activity of BAY 87-2243, suppression of HIF-1α protein levels, and reduction of HIF-1 target gene expression in vivo were demonstrated in a H460 xenograft model. BAY 87-2243 did not inhibit cell proliferation under standard conditions. However under glucose depletion, a condition favoring mitochondrial ATP generation as energy source, BAY 87-2243 inhibited cell proliferation in the nanomolar range. Further experiments revealed that BAY 87-2243 inhibits mitochondrial complex I activity but has no effect on complex III activity. Interference with mitochondrial function to reduce hypoxia-induced HIF-1 activity in tumors might be an interesting therapeutic approach to overcome chemo- and radiotherapy-resistance of hypoxic tumors.
Keywords: Antitumor activity; hypoxia; hypoxia-inducible factor-1; mitochondrial complex 1.
© 2013 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures




Similar articles
-
Prolyl hydroxylase 2 dependent and Von-Hippel-Lindau independent degradation of Hypoxia-inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition.BMC Cancer. 2012 Jul 17;12:293. doi: 10.1186/1471-2407-12-293. BMC Cancer. 2012. PMID: 22804960 Free PMC article.
-
Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway.Cancer Res. 2006 Jun 15;66(12):6264-70. doi: 10.1158/0008-5472.CAN-05-2519. Cancer Res. 2006. PMID: 16778202
-
Activation of hypoxia-induced transcription in normoxia.Exp Cell Res. 2005 May 15;306(1):180-91. doi: 10.1016/j.yexcr.2005.01.017. Epub 2005 Mar 19. Exp Cell Res. 2005. PMID: 15878343
-
Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: A one transcription factor (HIF-1) show?Biochim Biophys Acta. 2009 Apr;1795(2):162-72. doi: 10.1016/j.bbcan.2009.01.001. Epub 2009 Jan 22. Biochim Biophys Acta. 2009. PMID: 19344680 Free PMC article. Review.
-
Correlation of expression of hypoxia-related proteins with prognosis in oral squamous cell carcinoma patients.Oral Maxillofac Surg. 2012 Jun;16(2):189-96. doi: 10.1007/s10006-012-0335-8. Epub 2012 May 17. Oral Maxillofac Surg. 2012. PMID: 22592457 Review.
Cited by
-
Binding of Natural Inhibitors to Respiratory Complex I.Pharmaceuticals (Basel). 2022 Aug 31;15(9):1088. doi: 10.3390/ph15091088. Pharmaceuticals (Basel). 2022. PMID: 36145309 Free PMC article. Review.
-
TGF-β signaling in the tumor metabolic microenvironment and targeted therapies.J Hematol Oncol. 2022 Sep 17;15(1):135. doi: 10.1186/s13045-022-01349-6. J Hematol Oncol. 2022. PMID: 36115986 Free PMC article. Review.
-
NDUFS3 knockout cancer cells and molecular docking reveal specificity and mode of action of anti-cancer respiratory complex I inhibitors.Open Biol. 2022 Nov;12(11):220198. doi: 10.1098/rsob.220198. Epub 2022 Nov 9. Open Biol. 2022. PMID: 36349549 Free PMC article.
-
Essential roles of mitochondrial and heme function in lung cancer bioenergetics and tumorigenesis.Cell Biosci. 2018 Nov 2;8:56. doi: 10.1186/s13578-018-0257-8. eCollection 2018. Cell Biosci. 2018. PMID: 30410721 Free PMC article. Review.
-
Immune effects of PI3K/Akt/HIF-1α-regulated glycolysis in polymorphonuclear neutrophils during sepsis.Crit Care. 2022 Jan 28;26(1):29. doi: 10.1186/s13054-022-03893-6. Crit Care. 2022. PMID: 35090526 Free PMC article.
References
-
- Vaupel P, Thews O, Kelleher DK, Hoeckel M. Current status of knowledge and critical issues in tumor oxygenation. Results from 25 years research in tumor pathophysiology. Adv. Exp. Med. Biol. 1998;454:591–602. - PubMed
-
- Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. - PubMed
-
- Koh MY, Spivak-Kroizman T, Venturini S, Welsh S, Williams RR, Kirkpatrick DL, et al. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 2008;7:90–100. - PubMed
-
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 2003;9:677–684. - PubMed
-
- Kwon HC, Kim SH, Oh SY, Lee S, Kwon KA, Choi HJ, et al. Clinicopathological significance of p53, hypoxia-inducible factor 1alpha, and vascular endothelial growth factor expression in colorectal cancer. Anticancer Res. 2010;10:4163–4168. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

