Phenotypic modifications in ovarian cancer stem cells following Paclitaxel treatment
- PMID: 24403249
- PMCID: PMC3892380
- DOI: 10.1002/cam4.115
Phenotypic modifications in ovarian cancer stem cells following Paclitaxel treatment
Erratum in
- Cancer Med. 2013 Dec;2(6):987. Joo, Won Duk [added]
Abstract
Epithelial ovarian cancer (EOC) is the most lethal gynecologic malignancy. Despite initial responsiveness, 80% of EOC patients recur and present with chemoresistant and a more aggressive disease. This suggests an underlying biology that results in a modified recurrent disease, which is distinct from the primary tumor. Unfortunately, the management of recurrent EOC is similar to primary disease and does not parallel the molecular changes that may have occurred during the process of rebuilding the tumor. We describe the characterization of unique in vitro and in vivo ovarian cancer models to study the process of recurrence. The in vitro model consists of GFP+/CD44+/MyD88+ EOC stem cells and mCherry+/CD44-/MyD88- EOC cells. The in vivo model consists of mCherry+/CD44+/MyD88+ EOC cells injected intraperitoneally. Animals received four doses of Paclitaxel and response to treatment was monitored by in vivo imaging. Phenotype of primary and recurrent disease was characterized by quantitative polymerase chain reaction (qPCR) and Western blot analysis. Using the in vivo and in vitro models, we confirmed that chemotherapy enriched for CD44+/MyD88+ EOC stem cells. However, we observed that the surviving CD44+/MyD88+ EOC stem cells acquire a more aggressive phenotype characterized by chemoresistance and migratory potential. Our results highlight the mechanisms that may explain the phenotypic heterogeneity of recurrent EOC and emphasize the significant plasticity of ovarian cancer stem cells. The significance of our findings is the possibility of developing new venues to target the surviving CD44+/MyD88+ EOC stem cells as part of maintenance therapy and therefore preventing recurrence and metastasis, which are the main causes of mortality in patients with ovarian cancer.
Keywords: EMT; ovarian cancer stem cells; recurrence; slug.
© 2013 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
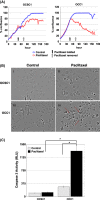
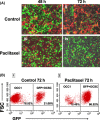
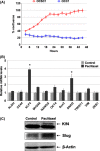

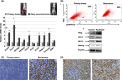

 day 0 designated as beginning of treatment;
day 0 designated as beginning of treatment;  day 12 is the fourth and final dose of Paclitaxel. *P < 0.05 compared to Control. **, *P < 0.001 compared to control no treatment (n = 10 animals per group).
day 12 is the fourth and final dose of Paclitaxel. *P < 0.05 compared to Control. **, *P < 0.001 compared to control no treatment (n = 10 animals per group).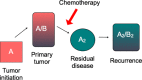
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. - PubMed
-
- Markman M. Optimal management of recurrent ovarian cancer. Int. J. Gynecol. Cancer. 2009;19(Suppl. 2):S40–S43. - PubMed
-
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

