Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer
- PMID: 24403266
- PMCID: PMC3892397
- DOI: 10.1002/cam4.137
Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer
Abstract
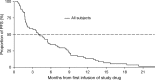
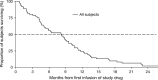
Tigatuzumab is the humanized version of the agonistic murine monoclonal antibody TRA-8 that binds to the death receptor 5 and induces apoptosis of human cancer cell lines via the caspase cascade. The combination of tigatuzumab and gemcitabine inhibits tumor growth in murine pancreatic xenografts. This phase 2 trial evaluated the efficacy of tigatuzumab combined with gemcitabine in 62 chemotherapy-naive patients with histologically or cytologically confirmed unresectable or metastatic pancreatic cancer. Patients received intravenous tigatuzumab (8 mg/kg loading dose followed by 3 mg/kg weekly) and gemcitabine (1000 mg/m(2) once weekly for 3 weeks followed by 1 week of rest) until progressive disease (PD) or unacceptable toxicity occurred. The primary end point was progression-free survival (PFS) at 16 weeks. Secondary end points included objective response rate (ORR) (complete responses plus partial responses), duration of response, and overall survival (OS). Safety of the combination was also evaluated. Mean duration of treatment was 18.48 weeks for tigatuzumab and 17.73 weeks for gemcitabine. The PFS rate at 16 weeks was 52.5% (95% confidence interval [CI], 39.3-64.1%). The ORR was 13.1%; 28 (45.9%) patients had stable disease and 14 (23%) patients had PD. Median PFS was 3.9 months (95% CI, 2.2-5.4 months). Median OS was 8.2 months (95% CI, 5.1-9.6 months). The most common adverse events related to tigatuzumab were nausea (35.5%), fatigue (32.3%), and peripheral edema (19.4%). Tigatuzumab combined with gemcitabine was well tolerated and may be clinically active for the treatment of chemotherapy-naive patients with unresectable or metastatic pancreatic cancer.
Keywords: Monoclonal antibodies; TNF-related apoptosis-inducing ligand; pancreatic cancer; phase 2; tigatuzumab.
© 2013 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;1:10–29. - PubMed
-
- National Cancer Institute. 2011. SEER stat fact sheets: pancreas. Available at http://seer.cancer.gov/statfacts/html/pancreas.html (accessed 2 April 2012)
-
- Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;6:2403–2413. - PubMed
-
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007;15:1960–1966. - PubMed
-
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB., III Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J. Clin. Oncol. 2002;15:3270–3275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

