The human papillomavirus E7 proteins associate with p190RhoGAP and alter its function
- PMID: 24403595
- PMCID: PMC3993551
- DOI: 10.1128/JVI.03263-13
The human papillomavirus E7 proteins associate with p190RhoGAP and alter its function
Abstract
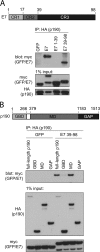
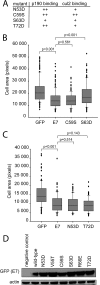
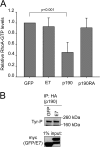
Using mass spectrometry, we identified p190RhoGAP (p190) as a binding partner of human papillomavirus 16 (HPV16) E7. p190 belongs to the GTPase activating protein (GAP) family and is one of the primary GAPs for RhoA. GAPs stimulate the intrinsic GTPase activity of the Rho proteins, leading to Rho inactivation and influencing numerous biological processes. RhoA is one of the best-characterized Rho proteins and is specifically involved in formation of focal adhesions and stress fibers, thereby regulating cell migration and cell spreading. Since this is the first report that E7 associates with p190, we carried out detailed interaction studies. We show that E7 proteins from other HPV types also bind p190. Furthermore, we found that conserved region 3 (CR3) of E7 and the middle domain of p190 are important for this interaction. More specifically, we identified two residues in CR3 of E7 that are necessary for p190 binding and used mutants of E7 with mutations of these residues to determine the biological consequences of the E7-p190 interaction. Our data suggest that the interaction of E7 with p190 dysregulates this GAP and alters the actin cytoskeleton. We also found that this interaction negatively regulates cell spreading on a fibronectin substrate and therefore likely contributes to important aspects of the HPV life cycle or HPV-induced tumorigenesis.
Importance: This study identifies p190RhoGAP as a novel cellular binding partner for the human papillomavirus (HPV) E7 protein. Our study shows that a large number of different HPV E7 proteins bind p190RhoGAP, and it identifies regions in both E7 and p190RhoGAP which are important for the interaction to occur. This study also highlights the likelihood that the E7-p190RhoGAP interaction may have important biological consequences related to actin organization in the infected cell. These changes could be an important contributor to the viral life cycle and during progression to cancer in HPV-infected cells. Importantly, this work also emphasizes the need for further study in a field which has largely been unexplored as it relates to the HPV life cycle and HPV-induced transformation.
Figures






References
-
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. 2000. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 92:709–720. 10.1093/jnci/92.9.709 - DOI - PubMed
-
- Masih AS, Stoler MH, Farrow GM, Wooldridge TN, Johansson SL. 1992. Penile verrucous carcinoma: a clinicopathologic, human papillomavirus typing and flow cytometric analysis. Mod. Pathol. 5:48–55 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

