A phosphatidylinositol transfer protein integrates phosphoinositide signaling with lipid droplet metabolism to regulate a developmental program of nutrient stress-induced membrane biogenesis
- PMID: 24403601
- PMCID: PMC3937096
- DOI: 10.1091/mbc.E13-11-0634
A phosphatidylinositol transfer protein integrates phosphoinositide signaling with lipid droplet metabolism to regulate a developmental program of nutrient stress-induced membrane biogenesis
Abstract
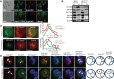
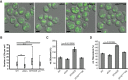
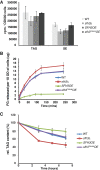
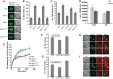
Lipid droplet (LD) utilization is an important cellular activity that regulates energy balance and release of lipid second messengers. Because fatty acids exhibit both beneficial and toxic properties, their release from LDs must be controlled. Here we demonstrate that yeast Sfh3, an unusual Sec14-like phosphatidylinositol transfer protein, is an LD-associated protein that inhibits lipid mobilization from these particles. We further document a complex biochemical diversification of LDs during sporulation in which Sfh3 and select other LD proteins redistribute into discrete LD subpopulations. The data show that Sfh3 modulates the efficiency with which a neutral lipid hydrolase-rich LD subclass is consumed during biogenesis of specialized membrane envelopes that package replicated haploid meiotic genomes. These results present novel insights into the interface between phosphoinositide signaling and developmental regulation of LD metabolism and unveil meiosis-specific aspects of Sfh3 (and phosphoinositide) biology that are invisible to contemporary haploid-centric cell biological, proteomic, and functional genomics approaches.
Figures





References
-
- Aitken JF, van Heusden GP, Temkin M, Dowhan W. The gene encoding the phosphatidylinositol transfer protein is essential for cell growth. J Biol Chem. 1990;265:4711–4717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

